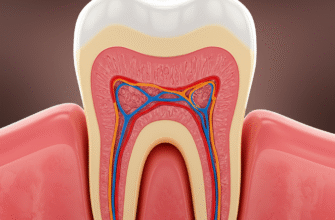
Deep within each tooth, shielded by layers of hard enamel and dentin, lies a vital, living tissue known as the dental pulp. Often referred to as the “nerve” of the tooth, this soft core is far more complex than a simple bundle of nerves. It’s a bustling hub of blood vessels, connective tissue, immune cells, and, crucially for our exploration, an intricate array of sensory receptors. These microscopic sentinels are responsible for the diverse range of sensations our teeth can experience, from the sharp zing of cold to the dull throb of an ache. Understanding these receptors offers a fascinating glimpse into how our bodies perceive and respond to the world around us, even at the level of a single tooth.
The Intricate Web of Innervation
The sensory experience of a tooth begins with nerve fibers that originate primarily from the trigeminal ganglion, a major collection of nerve cells. These fibers journey towards the jaw and enter each tooth through a tiny opening at the root tip, called the apical foramen. Once inside, they branch out extensively, forming a dense network, particularly in the coronal pulp (the part within the crown of the tooth) and just beneath the dentin layer. This subodontoblastic nerve plexus, often called Raschkow’s plexus, is a key area where sensory information is first gathered.
These nerve fibers are not all identical. They vary in size, in whether they are covered by a fatty insulating layer called myelin, and consequently, in the speed at which they transmit signals. This diversity is fundamental to the different types of sensations we can feel from our teeth. The pulp is a highly innervated tissue, meaning it’s packed with these neural connections, making it exquisitely sensitive to changes in its environment.
Dental pulp is densely innervated, primarily by sensory nerve fibers. These fibers originate from the trigeminal ganglion and enter the tooth through the apical foramen. This intricate network, especially Raschkow’s plexus, is responsible for the diverse range of sensations experienced by teeth, making them highly responsive organs.
Decoding Discomfort: The Nociceptors
The most well-known and perhaps most studied sensory receptors in dental pulp are nociceptors. These are specialized nerve endings that respond to stimuli that have the potential to cause tissue damage, or stimuli that are already causing damage. The brain interprets signals from nociceptors as pain. Pain, while unpleasant, serves a vital protective role, alerting us to problems that need attention. In the dental pulp, nociceptors are the primary guardians, signaling issues like decay approaching the pulp, inflammation, or physical trauma.
A-Delta Fibers: The Sharp Shooters
Among the nociceptive fibers, A-delta (Aδ) fibers are notable. These are relatively thin and lightly myelinated. Myelin acts like insulation around an electrical wire, allowing nerve impulses to travel much faster. Because of this, A-delta fibers are responsible for transmitting sensations of sharp, well-localized pain. Think of the quick, intense zing you might feel when biting into something unexpectedly hard or when cold air hits a sensitive tooth. These fibers are particularly responsive to mechanical stimuli (like pressure or probing) and to rapid changes in temperature, especially cold. Their rapid response is crucial for immediate withdrawal from a potentially harmful stimulus.
C-Fibers: The Dull Ache Architects
In contrast to the swift A-delta fibers, C-fibers are unmyelinated and therefore conduct nerve impulses much more slowly. They are typically associated with a different quality of pain: a dull, throbbing, burning, or aching sensation that can be more diffuse and harder to pinpoint. C-fibers are often polymodal, meaning they can be activated by a variety of stimuli, including intense mechanical pressure, extreme temperatures (both hot and cold), and, importantly, chemical mediators released during inflammation. When the pulp becomes inflamed (a condition often stemming from deep decay or injury), substances like bradykinin, prostaglandins, and histamine are released. These substances can directly activate C-fibers or make them more sensitive to other stimuli, contributing to the persistent, often gnawing pain associated with a toothache.
Feeling the Pressure: Are Mechanoreceptors in the Mix?
While pain is the dominant sensation originating from the pulp, there’s ongoing research into whether other types of sensory receptors, specifically mechanoreceptors, play a significant role. Mechanoreceptors are designed to respond to innocuous mechanical stimuli like touch, pressure, and vibration. In most parts of the body, they provide information about texture, shape, and movement. In teeth, the periodontal ligament surrounding the root is rich in mechanoreceptors, giving us a fine sense of tooth movement and biting force.
The presence and function of true, low-threshold mechanoreceptors *within* the pulp itself are less clear-cut than nociceptors. Some studies have identified structures resembling Ruffini endings (which respond to sustained pressure and stretch) or Pacinian-like corpuscles (responding to vibration and deep pressure), but their density is low compared to nociceptors. It’s plausible that some A-delta fibers, while primarily nociceptive, might also respond to non-painful mechanical forces to some degree. The primary sensation of pressure when chewing is largely attributed to the periodontal ligament, but the pulp’s potential contribution to discerning subtle forces or internal pressure changes remains an area of investigation.
The Hot and Cold of It: Thermoreceptors and TRP Channels
Sensitivity to temperature is a hallmark of dental sensation, and this isn’t solely the domain of pain fibers. Specialized molecules known as Transient Receptor Potential (TRP) channels are heavily involved in detecting thermal changes. These channels are proteins embedded in the membranes of nerve endings that open or close in response to specific temperatures, allowing ions to flow and trigger a nerve signal.
Several types of TRP channels have been identified in dental pulp afferents:
- TRPV1 (Transient Receptor Potential Vanilloid 1): This channel is famously activated by heat (typically above 42-43°C, or 108-109°F) and by capsaicin, the pungent compound in chili peppers. It contributes to the sensation of burning pain from hot stimuli.
- TRPM8 (Transient Receptor Potential Melastatin 8): This is the primary cold sensor, activated by cool to cold temperatures (typically below 25-28°C, or 77-82°F) and also by cooling compounds like menthol. It’s a key player in the sharp sensation experienced with cold drinks or foods.
- TRPA1 (Transient Receptor Potential Ankyrin 1): This channel is a bit more complex. It can be activated by noxious cold, as well as by a wide range of chemical irritants, including compounds found in mustard oil, garlic, and cinnamon, and also by inflammatory mediators. It often contributes to pain hypersensitivity.
The interplay of these TRP channels on different nerve fibers (both A-delta and C-fibers can express them) allows the tooth to not just sense “hot” or “cold” as painful, but also to discern gradations of temperature, at least to some extent, before the pain threshold is reached.
The Symphony of Sensation: How Dentin Plays Its Part
One of the enduring questions in dental sensory physiology is how stimuli applied to the outer surface of dentin (the layer beneath the enamel) can evoke a sensation, given that the main nerve plexus is located in the pulp. Dentin itself is not directly innervated throughout its full thickness, though some nerve fibers do penetrate a short distance into the dentinal tubules near the pulp.
The most widely accepted explanation is the hydrodynamic theory. Dentin is permeated by thousands of microscopic channels called dentinal tubules, which run from the pulp-dentin junction towards the enamel-dentin junction. These tubules are filled with dentinal fluid. According to the hydrodynamic theory, stimuli like temperature changes, osmotic shifts (e.g., from sugary substances), or air drying can cause this fluid within the tubules to move rapidly. This fluid movement, whether inward or outward, is thought to distort the nerve endings located at the pulp-dentin border or within the inner parts of the tubules. This mechanical distortion then activates the A-delta fibers, leading to the characteristic sharp, brief pain often associated with dentin hypersensitivity.
So, while the receptors are in or near the pulp, the unique structure of dentin acts as a transducer, converting various external stimuli into a mechanical force that the pulpal nerves can detect.
Beyond Simple Wires: Modulation and Complexity
The sensory system of the dental pulp is not a static network of wires. It’s a dynamic environment where the sensitivity of receptors can be significantly altered. During inflammation, for instance, the chemical soup released by immune cells and damaged tissue can lead to peripheral sensitization. This means that nociceptors become more responsive: they might fire in response to stimuli that wouldn’t normally cause pain (allodynia), or they might respond more intensely to painful stimuli (hyperalgesia). This is why a tooth with an inflamed pulp can become exquisitely tender to even slight temperature changes or gentle touch.
Neuropeptides, such as Substance P and Calcitonin Gene-Related Peptide (CGRP), released from the sensory nerve endings themselves, also play a complex role. They can contribute to neurogenic inflammation (inflammation caused by nerve activity) by affecting blood vessels and immune cells, further sensitizing the nerve endings in a feedback loop. This highlights the intricate communication between the nervous system and the other cellular components of the pulp.
In essence, the dental pulp is equipped with a sophisticated detection system, capable of alerting us to a wide range of environmental challenges. From the rapid alarm signals of A-delta fibers to the persistent warnings of C-fibers, and the nuanced temperature information provided by TRP channels, these sensory receptors are vital for protecting the tooth’s integrity. Exploring their functions continues to reveal the remarkable complexity packed within the living core of each tooth.