Your smile’s first line of defense, that gleaming outer layer of your teeth, is a true marvel of natural engineering: tooth enamel. It’s the hardest substance in the human body, tougher than bone, designed to withstand the immense pressures of chewing day in and day out. Yet, for all its incredible strength, enamel possesses a curious vulnerability – it’s surprisingly brittle. This fascinating paradox, this combination of robust resilience and delicate fragility, defines enamel’s character and dictates how we must care for our teeth. How can something so tough also be so prone to chipping and cracking? The answer lies deep within its intricate composition and microscopic architecture.
The Mighty Mineral Shield: Composition of Enamel
At its very core, tooth enamel is overwhelmingly mineral. About 96 percent of its substance is made up of tiny crystals of a calcium phosphate mineral called hydroxyapatite. This isn’t just any hydroxyapatite; it’s a highly organized, densely packed form that gives enamel its signature hardness. Imagine microscopic, elongated crystals, intricately arranged to form a robust barrier. These crystals are the building blocks that stand up to the forces of biting and grinding, providing that incredible surface strength we rely on for every meal.
While minerals dominate, the remaining fraction, a mere 4 percent or so, consists of water and organic materials. This small organic component, primarily unique proteins like amelogenins and enamelins (present mostly during enamel formation), isn’t enough to lend much flexibility. Unlike bone, which has a significant collagen matrix providing toughness and resilience against fracture, enamel sacrifices this pliability for sheer surface hardness. This compositional imbalance is a key reason behind its brittleness.
Perhaps one of the most critical aspects of enamel’s composition is what it lacks: living cells. Once your teeth are fully formed, the cells responsible for creating enamel (ameloblasts) are gone. This means enamel cannot regenerate or repair itself if it’s damaged by decay, wear, or trauma. What you have is what you get, making its preservation a lifelong endeavor. This acellular nature distinguishes it sharply from other hard tissues in the body like bone, which can heal and remodel.
Woven for Strength: The Microarchitecture
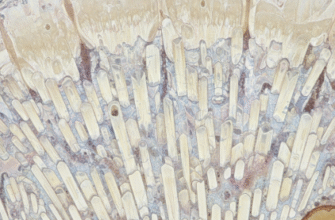
The incredible hardness of enamel isn’t just due to its mineral content; it’s also a testament to its sophisticated microscopic structure. Think of it not as a simple, uniform block of mineral, but as a complex, woven material, meticulously engineered for durability. The primary structural units are called enamel rods or enamel prisms. Millions of these tiny, tightly packed rods, typically running from the underlying dentin surface towards the tooth’s exterior, make up the bulk of the enamel layer.
These rods are not isolated structures. They are cemented together by something called interrod enamel, which has a slightly different crystal orientation. This subtle difference in how the hydroxyapatite crystals are aligned within the rods versus between them helps to deflect cracks and dissipate stress. It’s a bit like how fibers in a composite material are arranged to maximize strength. The boundaries between these rods, known as prism sheaths, are richer in organic material, though still very sparse, and can sometimes be initial pathways for demineralization.
The Rod Arrangement: A Masterclass in Engineering
The arrangement of these enamel rods is particularly fascinating and crucial for enamel’s function. Often described as having a keyhole or paddle shape in cross-section, these rods are bundled together in a way that maximizes density and minimizes weak points. Near the surface of the tooth, they tend to run roughly perpendicular to the tooth surface, which is ideal for resisting direct biting forces. Deeper, near the junction with the dentin, their arrangement can be more complex, even decussating or weaving, which helps to prevent cracks that start at the surface from easily propagating straight through the entire enamel layer.
This intricate organization means that enamel can withstand incredible compressive forces – the kind you exert when you bite down hard. The tightly packed, well-oriented crystals in the rods bear the brunt of this load. However, this same highly ordered, rigid structure contributes to its brittleness when subjected to shear or tensile stresses, or sharp impacts.
The Achilles’ Heel: Why So Brittle?
So, if enamel is so densely packed with super-hard mineral crystals, why does it chip or crack? The answer, as hinted, lies in what it sacrifices for that hardness: flexibility. The very high degree of mineralization, that impressive 96 percent hydroxyapatite, means there’s very little room for organic material that could absorb shocks or allow the structure to bend even slightly under stress. Think of materials like glass or ceramic; they are incredibly hard but will shatter under a sharp impact because they cannot deform to absorb the energy.
Enamel behaves similarly. It lacks the significant protein network, particularly collagen, that gives materials like bone their toughness (resistance to fracture). Bone can bend before it breaks, to a certain extent, thanks to its roughly 30 percent organic content. Enamel, with its minimal organic matrix, is all about rigid strength. This makes it superb at resisting wear and surface indentation, but when the force exceeds its limit or comes from an awkward angle, it tends to fracture rather than deform. It’s a trade-off: extreme hardness for an inherent brittleness.
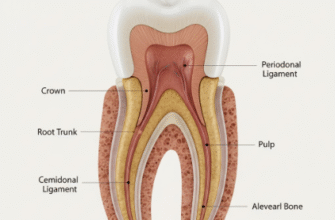
The Dynamic Duo: Enamel and Dentin
Nature, however, has a clever solution to partly compensate for enamel’s brittleness. Beneath the hard enamel shell lies a layer of dentin, which forms the bulk of the tooth. Dentin is also a hard, mineralized tissue, but it’s significantly less mineralized than enamel (around 70 percent mineral) and contains a higher proportion of organic material, including collagen fibers. This makes dentin considerably more elastic and resilient – it can absorb more shock and flex slightly under load.
This partnership is crucial. The softer, more forgiving dentin acts like a cushion, supporting the brittle enamel layer above it. When you bite down, the force is transmitted through the rigid enamel to the more resilient dentin, which can deform slightly to dissipate some of the stress. Without this supportive dentinal foundation, enamel would be far more prone to fracturing under normal chewing pressures. It’s a composite structure, where the properties of each layer complement the other.
The interface between these two layers, the dentinoenamel junction (DEJ), is also a fascinating and complex structure. It’s not a simple flat boundary but a scalloped or wavy interface that helps to lock the two tissues together and resist shearing forces. This intricate junction also plays a role in preventing cracks that start in the enamel from easily propagating into the dentin, thereby helping to preserve the tooth’s overall integrity.
It is a critical point to remember that tooth enamel, for all its strength, possesses no living cells once tooth formation is complete. This means that any enamel lost to decay, erosion, or fracture cannot be naturally regenerated by your body. This irreplaceability underscores the importance of preserving the enamel you have through careful oral hygiene and dietary choices.
When the Shield Cracks: Consequences of Brittleness
Despite the supportive dentin, enamel’s inherent brittleness means it’s still susceptible to damage. Sharp impacts, such as biting down on an unexpected olive pit or an ice cube, or trauma from an accident, can cause the enamel to chip or fracture. These fractures can range from minor craze lines (tiny, superficial cracks that may not cause immediate problems but can stain) to more significant chips that expose the underlying dentin, leading to sensitivity and an increased risk of decay.
Chronic stresses, like those from teeth grinding (bruxism) or clenching, can also lead to micro-cracks over time. While enamel is excellent at resisting compressive forces, these habitual actions can introduce tensile and shear stresses that it’s less equipped to handle. Over time, these tiny cracks can propagate, potentially leading to larger fractures or cusps breaking off. The way enamel fails is characteristic of brittle materials – a sudden crack rather than a slow bending or tearing.
Factors That Test Enamel’s Limits
Beyond direct physical trauma, enamel faces other challenges that can exploit its properties. Acid erosion is a major one. Acids from dietary sources (like citrus fruits, sodas, and wine) or those produced by bacteria in dental plaque can dissolve the hydroxyapatite crystals. This demineralization weakens the enamel structure, making it softer and more susceptible to wear and fracture. While not directly related to its brittleness, a thinned or softened enamel layer is much easier to damage physically.
Mechanical wear, or attrition (tooth-on-tooth contact) and abrasion (from foreign objects like overly aggressive brushing or abrasive toothpastes), also gradually grinds away the enamel surface. Because enamel cannot repair itself, this wear is cumulative. The combination of chemical weakening from acids and subsequent mechanical wear can accelerate enamel loss significantly, testing the limits of even its impressive hardness and eventually exposing its brittle nature to more direct forces as it thins.
Tooth enamel stands as a remarkable example of biological material science – a substance engineered for incredible hardness to perform one of life’s most essential functions: eating. Its densely packed mineral crystals provide unparalleled resistance to the daily grind. Yet, this same specialization comes with an inherent brittleness, a fragility that reminds us of the delicate balance within our bodies. Understanding this dual nature of strength and vulnerability helps us appreciate not only the marvel of enamel but also the continuous need to protect this irreplaceable shield.