That bright, dazzling smile – many of us want one, and often, achieving it involves some form of teeth whitening. But have you ever wondered what is actually happening on a microscopic level when you use a whitening product? It is not just magic; it is a fascinating interplay of chemistry and biology. Understanding the science behind teeth whitening can help you appreciate the process, understand its limitations, and make more informed choices if you are considering it.
The Canvas: Understanding Your Tooth Structure
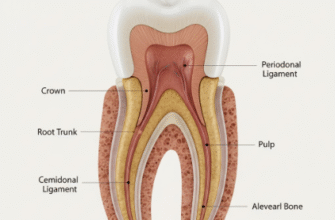
Before we dive into how whitening works, let us get familiar with the structure of a tooth. Think of it in layers. The outermost layer is the enamel. This is the hardest substance in the human body, even tougher than bone. Enamel is mostly made of a mineral called hydroxyapatite, which forms a crystalline lattice structure. While incredibly strong, enamel is semi-porous, meaning it has microscopic channels or pores. This porosity is key to how stains accumulate and how whitening agents can penetrate.
Beneath the enamel lies the dentin. Dentin is softer than enamel and has a more yellowish hue. It is made up of tiny tubules that run from the pulp (the innermost part of the tooth containing nerves and blood vessels) towards the enamel. The natural color of your dentin significantly influences the overall shade of your teeth, especially if your enamel is thin or has worn down over time. The thickness and translucency of your enamel, combined with the color of your dentin, determine the baseline color of your teeth.
Why Teeth Get Stained: The Culprits
Tooth discoloration can broadly be categorized into two types: extrinsic and intrinsic stains.
Extrinsic Stains
These are the bad boys most people think about when they consider whitening. Extrinsic stains sit on the surface of the enamel. They are caused by chromogens – intensely pigmented molecules – found in certain foods, drinks, and habits. Common culprits include:
- Coffee and tea (tannins)
- Red wine
- Dark sodas
- Berries and other deeply colored fruits
- Tomato-based sauces
- Tobacco (tar and nicotine)
These chromogens latch onto the enamel surface and can also get trapped in that microscopic porous network. Over time, they accumulate, leading to a yellowed or dingy appearance. Poor oral hygiene can exacerbate this, as plaque buildup can also attract and hold onto stains.
Intrinsic Stains
Intrinsic stains are a different beast altogether. These stains are located within the tooth structure, either in the enamel itself or, more commonly, in the dentin. Because they are deeper, they are generally more resistant to simple surface whitening methods. Causes of intrinsic staining include:
- Medications: Certain antibiotics, like tetracycline and doxycycline, if taken during tooth development (childhood), can bind to the calcium in developing teeth, causing permanent grayish or brownish bands. Antihistamines, antipsychotic drugs, and medications for high blood pressure can also sometimes cause tooth discoloration.
- Trauma: An injury to a tooth can cause bleeding within the pulp, and as the blood breaks down, iron pigments can seep into the dentin, darkening the tooth from the inside out.
- Fluorosis: Excessive fluoride intake during tooth formation can lead to dental fluorosis, which can manifest as faint white lines, streaks, or in more severe cases, brown spots or pitting on the enamel.
- Aging: As we age, enamel naturally thins, allowing more of the yellowish dentin to show through. Dentin itself can also produce more secondary dentin over time, which can be darker.
- Genetics: Some people are simply born with enamel that is naturally brighter or thicker than others.
The Chemical Warfare: How Whitening Agents Work
The vast majority of effective teeth whitening products, whether over-the-counter (OTC) or professionally administered, rely on one of two key chemical agents: hydrogen peroxide or carbamide peroxide. These are both oxidizing agents, and that is where the magic happens.
When these peroxides come into contact with your teeth, they initiate an oxidation reaction. Let us break that down:
- Breakdown: Hydrogen peroxide (H2O2) itself is unstable and readily breaks down. Carbamide peroxide is a more stable compound that, when it comes into contact with water (like saliva), breaks down into hydrogen peroxide and urea. So, carbamide peroxide is essentially a slower-releasing delivery system for hydrogen peroxide. Typically, a 10% carbamide peroxide solution is roughly equivalent to a 3.5% hydrogen peroxide solution in terms of its ultimate peroxide delivery.
- Free Radicals Unleashed: The hydrogen peroxide then decomposes further, producing highly reactive molecules called free radicals (often oxygen radicals like the hydroxyl radical •OH, or perhydroxyl radical •OOH). These free radicals are the workhorses of the whitening process.
- Attacking Chromogens: Chromogens, those pesky stain molecules we talked about earlier, are complex organic molecules with conjugated double bonds. These double bonds are what allow them to absorb light and appear colored. The free radicals are highly reactive and attack these double bonds, breaking them down into smaller, simpler, and less pigmented molecules. By cleaving these bonds, the chromogens lose their ability to absorb light in the same way, making them colorless or much lighter.
Essentially, the peroxide agents diffuse through the porous enamel and into the dentin, where they chemically scrub away the stain molecules. The smaller, colorless molecules are then naturally washed away or remain unseen, resulting in a whiter appearance. The effectiveness depends on the concentration of the peroxide and the duration of contact with the teeth.
The core mechanism of teeth whitening involves oxidation. Peroxide-based agents release free radicals that penetrate the tooth enamel. These radicals break the chemical bonds of discolored chromogen molecules, rendering them colorless and thus lightening the tooth’s appearance.
Methods of Whitening: A Scientific Perspective
There is a spectrum of whitening options available, each employing these chemical principles in different ways.
Over-the-Counter (OTC) Products
These are readily available and generally contain lower concentrations of peroxide.
- Whitening Toothpastes: Most whitening toothpastes do not contain significant levels of peroxide. Instead, they primarily work through mechanical abrasion, using mild abrasives like hydrated silica, calcium carbonate, or dicalcium phosphate to scrub away surface extrinsic stains. Some may contain very low concentrations of chemical agents like sodium hexametaphosphate to help prevent new stains or tiny amounts of blue covarine, which deposits a thin film on teeth, creating an optical illusion of whiteness. Their effect is mostly limited to surface stains.
- Whitening Strips: These are thin, flexible plastic strips coated with a peroxide-based gel (usually hydrogen peroxide, sometimes carbamide peroxide). Concentrations typically range from 6% to 14% hydrogen peroxide. The strips are applied directly to the teeth for a set period, usually 30 minutes to an hour, once or twice a day for a couple of weeks. The peroxide is held against the tooth surface, allowing it to diffuse into the enamel.
- Whitening Gels and Tray Systems (OTC): These involve a generic (boil-and-bite) tray that you fill with a peroxide gel (again, hydrogen or carbamide peroxide, often in the 10% to 22% carbamide peroxide range, which translates to about 3.5% to 8% hydrogen peroxide). The tray helps keep the gel in contact with the teeth.
Professional Whitening Options
Performed or prescribed by a dentist, these methods generally use higher concentrations of peroxide and offer more controlled application.
- In-Office Whitening: This is the fastest way to achieve significant whitening. It involves the application of a high-concentration peroxide gel (typically 15% to 43% hydrogen peroxide) directly to the teeth by a dental professional. Before application, your gums and soft tissues are protected with a rubber dam or a protective gel. Often, a special light or laser is used in conjunction with the gel. The science on whether these lights significantly accelerate or enhance the chemical whitening process itself is debated; some studies suggest the primary effect of the light might be heat, which can speed up the decomposition of peroxide, while others show minimal additional benefit over the chemical action alone. The high concentration allows for noticeable results in a single visit of about an hour.
- Professionally Dispensed Take-Home Kits: These kits feature custom-fitted trays made by your dentist from impressions of your teeth. The custom fit ensures maximum contact between the whitening gel (usually carbamide peroxide in concentrations from 10% to 38%, or hydrogen peroxide equivalents) and your teeth, while minimizing gel leakage onto the gums. You use these trays at home for a specified period each day or overnight, typically for one to two weeks.
Factors Influencing Whitening Efficacy and Potential Issues
Several factors influence how well whitening works and what side effects might occur.
Concentration and Contact Time
Higher peroxide concentrations and longer contact times generally lead to more dramatic and faster whitening. However, they also increase the risk of side effects like tooth sensitivity and gum irritation. This is why high-concentration products are typically administered or supervised by dental professionals who can protect soft tissues and monitor the process.
Tooth Sensitivity
This is the most common side effect. It is believed to occur because the peroxide can penetrate the enamel and dentin, reaching the dentinal tubules that lead to the tooth’s pulp (nerve). This can cause temporary inflammation or irritation of the pulp, experienced as sensitivity to cold, air, or sweet things. The urea in carbamide peroxide systems might have a slight buffering effect and its slower release of hydrogen peroxide is sometimes associated with less initial sensitivity for some individuals. Sensitivity is usually temporary and subsides after treatment stops or with the use of desensitizing toothpastes containing potassium nitrate.
Gum (Gingival) Irritation
If the whitening gel comes into contact with the gums, it can cause a chemical burn, leading to temporary whitening, irritation, or soreness of the soft tissues. This is why professional applications involve gum protection, and why custom-fitted trays are superior to ill-fitting OTC trays in preventing gel leakage.
The Limitations: What Whitening Can’t Fix
It is crucial to understand that whitening agents only work on natural tooth structure. They do not whiten dental restorations like fillings, veneers, crowns, or bonding. If you have these in your front teeth, whitening your natural teeth could lead to a noticeable mismatch in color. Intrinsic stains, especially those caused by tetracycline or severe fluorosis, are also much more difficult to whiten significantly with standard peroxide treatments and may require alternative cosmetic dental procedures.
Teeth whitening is not suitable for everyone. Individuals with active gum disease, cavities, worn enamel, or highly sensitive teeth should consult their dentist before attempting any whitening procedure. Pregnant or lactating women are also generally advised to postpone whitening treatments.
The Fad of “Natural” Whiteners: A Scientific Look
You will often see claims about natural ingredients whitening teeth, such as activated charcoal, baking soda, or fruits like strawberries and lemons.
- Activated Charcoal: This is highly abrasive. While it might scrub off some surface stains, it can also wear away your enamel over time, making teeth appear more yellow as the dentin shows through, and potentially increasing sensitivity. There is little robust scientific evidence to support its efficacy or safety for long-term whitening.
- Baking Soda (Sodium Bicarbonate): Baking soda is mildly abrasive and can help remove some surface stains, much like a whitening toothpaste. It does not, however, change the intrinsic color of your teeth as peroxides do. Used excessively, its abrasiveness could also be a concern.
- Fruit Acids (e.g., Lemon, Strawberry): Fruits like lemons and strawberries contain acids (citric acid, malic acid). While rubbing them on your teeth might seem to make them appear brighter initially, this is often due to the acid eroding a very thin layer of enamel. Repeated use can significantly damage enamel, leading to increased sensitivity and a higher risk of cavities. This is not a recommended or safe method for whitening.
True whitening, in the sense of changing the intrinsic color of the tooth, relies on the chemical oxidation process that only agents like peroxides can provide effectively and relatively safely when used correctly.
Maintaining Your Brighter Smile
Once you have achieved your desired shade, maintaining it involves good oral hygiene practices – brushing twice a day, flossing daily – and minimizing exposure to staining agents. Rinsing your mouth with water after consuming coffee, tea, or red wine can help. Depending on the method used and your lifestyle, touch-up treatments may be needed periodically.
The science of teeth whitening is a clear demonstration of targeted chemistry at work, transforming smiles by altering the very molecules that cause discoloration. While the allure of a brighter smile is strong, understanding the mechanisms, benefits, and potential drawbacks allows for a more informed approach to achieving it, ideally with guidance from your dental professional who can help determine the most appropriate and safe path for your individual needs.