The creation of a tooth, a process known as odontogenesis, is a marvel of biological engineering. It’s not simply a matter of cells multiplying; rather, it’s an intricate dance of cellular communication, differentiation, and organization, primarily orchestrated through a continuous dialogue between two fundamental tissue types: the oral epithelium and the underlying neural crest-derived ectomesenchyme. These interactions are not random; they are precisely guided by a symphony of signaling molecules, with growth factors playing the lead conductor roles, ensuring each step, from the earliest initiation to the final hardening of enamel, occurs with remarkable precision. Understanding these growth factors offers profound insights into how our teeth form and, perhaps more critically, how they might be encouraged to repair themselves.
The Conductors: What Are Growth Factors?
So, what exactly are these pivotal molecules? Growth factors are naturally occurring proteins or, in some cases, steroid hormones that act as signaling messengers between cells. Their primary function is to stimulate or modulate cellular growth, proliferation, healing, migration, and cellular differentiation. They are the body’s internal communicators, relaying messages that tell cells when to divide, what to become, or when to build new tissue. They typically exert their effects by binding to specific receptors on the surface of target cells, much like a key fitting into a specific lock. This binding event triggers a cascade of intracellular events, a chain reaction inside the cell that ultimately alters gene expression and cellular behavior. Without these sophisticated signaling mechanisms, complex structures like teeth simply couldn’t form with such accuracy or possess the inherent capacity for limited repair.
Key Growth Factor Families Steering Tooth Development
Tooth development is a multi-act play, and several families of growth factors take center stage at different times, often interacting with each other. Their expression is tightly regulated, both spatially and temporally, ensuring the right signals are delivered to the right cells at the right moment.
Fibroblast Growth Factors (FGFs)
The FGF family is a large group of signaling proteins crucial from the very outset of tooth development. FGFs, such as FGF4, FGF8, FGF9, and FGF10, are heavily involved in the initiation of tooth formation, signaling the oral epithelium to thicken and form the dental lamina, the precursor to the tooth germ. They play vital roles in promoting cell proliferation in both epithelial and mesenchymal compartments. Later in development, FGFs contribute to the complex shaping, or morphogenesis, of the tooth, influencing the folding of the enamel organ that dictates the eventual crown pattern. Their ability to stimulate angiogenesis (new blood vessel formation) is also critical for supplying the developing tooth with nutrients and oxygen.
Bone Morphogenetic Proteins (BMPs)
As part of the larger Transforming Growth Factor-beta (TGF-β) superfamily, Bone Morphogenetic Proteins (BMPs) are indispensable for skeletal and dental development. BMP2, BMP4, and BMP7 are particularly prominent in odontogenesis. BMP4, for instance, is expressed in the early dental mesenchyme and signals back to the epithelium, a classic example of reciprocal induction. BMPs are essential for the differentiation of odontoblasts (dentin-forming cells) and ameloblasts (enamel-forming cells). They regulate cusp patterning – the specific shapes of the bumps on our molars – and are crucial for the deposition and mineralization of both dentin and enamel. The precise balance and location of BMP signaling dictate the fate of dental cells and the architecture of the hard tissues.
Transforming Growth Factor-beta (TGF-β) Superfamily
Beyond BMPs, other members of the TGF-β superfamily, including TGF-β1, TGF-β2, and TGF-β3, as well as activins and inhibins, have significant roles. TGF-β1, for example, is abundant in the dentin matrix and is thought to be released during tooth injury, playing a role in signaling repair processes. These factors are broadly involved in controlling cell proliferation, differentiation, apoptosis (programmed cell death, which is important for shaping tissues), and extracellular matrix production. Their effects can be context-dependent, sometimes promoting growth and other times inhibiting it, showcasing the complexity of their regulatory functions.
Sonic Hedgehog (SHH)
The Sonic Hedgehog (SHH) signaling pathway is another ancient and highly conserved pathway critical for embryonic development, including that of teeth. SHH is primarily expressed in the dental epithelium and acts as a mitogen, stimulating cell division. It plays a crucial role in establishing the sites of tooth formation (dental placodes) and in the patterning of the tooth crown. SHH signaling is involved in maintaining the proliferative activity of epithelial stem cells within structures like the cervical loop, which is essential for continuous tooth growth in species with ever-growing incisors, and for root formation in human teeth. Its interaction with other signaling pathways, like BMPs and FGFs, is vital for coordinating different aspects of tooth morphogenesis.
Wingless-related integration site (WNT) Signaling
The WNT signaling pathway is fundamental to numerous developmental processes, and tooth development is no exception. WNT signals, such as Wnt10a and Wnt10b, are involved in the very early stages of tooth initiation, helping to define where teeth will form along the jaw. They also play roles in cell fate decisions, proliferation, and polarity. Dysregulation of WNT signaling has been linked to various dental anomalies, including missing teeth (agenesis) or malformed teeth, highlighting its critical importance. The pathway contributes to the differentiation of ameloblasts and is involved in maintaining the stem cell niche within the tooth.
Epidermal Growth Factor (EGF) Family
The EGF family, including EGF itself and Transforming Growth Factor-alpha (TGF-α), primarily influences cell proliferation and differentiation. EGF receptors are found on both epithelial and mesenchymal cells of the developing tooth. While perhaps not as extensively studied in early tooth patterning as FGFs or BMPs, EGF signaling contributes to the growth and maturation of the enamel organ and the differentiation of ameloblasts. It also has roles in salivary gland development, which is closely related to the oral environment of the teeth.
Scientific understanding confirms that tooth development isn’t driven by a single master growth factor. Instead, it’s a highly coordinated process involving numerous growth factors acting in specific sequences and combinations. These factors often influence each other, creating intricate signaling networks essential for the precise formation of dental tissues and their subsequent maintenance.
Orchestrating Development: Growth Factors Stage by Stage
The journey from a simple epithelial thickening to a fully formed tooth involves several distinct morphological stages, each meticulously regulated by growth factors.
Initiation and Placode Formation
The very first sign of a tooth begins with signals, including FGF8 and SHH from the oral epithelium, which induce the underlying mesenchyme. This leads to the formation of the dental placode, a localized thickening of the epithelium. WNT signaling is also crucial here, determining the precise sites where teeth will emerge.
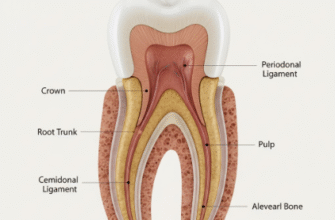
Morphogenesis: Bud, Cap, and Bell Stages
The placode then proliferates and invaginates into the mesenchyme, forming the bud stage. Growth factors like BMPs and FGFs drive this proliferation and initial shaping. As development proceeds to the cap stage, the epithelial component, now called the enamel organ, envelops the condensed dental mesenchyme (the dental papilla). Complex signaling centers, like the primary enamel knot, emerge at this stage, expressing a cocktail of growth factors (SHH, BMPs, FGFs) that dictate cusp formation and further morphogenesis. The bell stage is characterized by advanced differentiation and shaping. The enamel organ differentiates into distinct layers, and the overall form of the tooth crown becomes clearly defined. The interplay between epithelial and mesenchymal growth factors (e.g., BMP4 from mesenchyme influencing epithelial differentiation, and FGFs from epithelium influencing mesenchymal development) is paramount.
Cytodifferentiation: Birth of Specialized Cells
During the late bell stage, cells within the enamel organ and dental papilla undergo terminal differentiation. Inner enamel epithelial cells differentiate into ameloblasts (enamel-forming cells), while peripheral cells of the dental papilla differentiate into odontoblasts (dentin-forming cells). This critical step is heavily influenced by BMPs, TGF-β1, FGFs, and SHH, which trigger specific gene expression programs leading to these specialized cell types.
Matrix Formation and Mineralization
Once differentiated, odontoblasts begin to secrete dentin, the primary hard tissue of the tooth. Shortly after, ameloblasts secrete enamel, the hardest substance in the human body. Growth factors continue to play a role here, not just in maintaining the differentiated state of these cells but also in regulating the composition and organization of the extracellular matrix they produce. TGF-β1, stored in large quantities within the dentin matrix, is a key example.
Root Development
After crown formation is largely complete, root development begins. This process is guided by Hertwig’s Epithelial Root Sheath (HERS), an extension of the cervical loop of the enamel organ. HERS cells signal the adjacent dental papilla cells to differentiate into root odontoblasts, which then form root dentin. Growth factors like SHH and BMPs are involved in HERS function and the induction of root odontoblasts. The eventual breakdown of HERS allows dental follicle cells to migrate and differentiate into cementoblasts (forming cementum), osteoblasts (forming alveolar bone), and fibroblasts (forming the periodontal ligament).
Growth Factors in Tooth Repair and the Glimmer of Regeneration
Our teeth possess a limited, yet fascinating, capacity for natural repair, largely mediated by growth factors.
Natural Repair: Tertiary Dentin Formation
When a tooth is injured, for example, by dental caries or trauma, the underlying pulp tissue can respond by forming reparative or tertiary dentin. This is a protective mechanism to wall off the injury and preserve pulp vitality. This process is initiated by signaling molecules, including growth factors, that are released from the dentin matrix when it’s damaged or dissolved by bacterial acids. TGF-β1, BMPs, and FGFs sequestered within the dentin are liberated and can diffuse towards the pulp, stimulating progenitor cells to differentiate into new odontoblast-like cells that deposit this reparative dentin.
The Research Landscape: Exploring Growth Factor Potential
The inherent role of growth factors in natural tooth repair has spurred considerable research into their potential therapeutic applications in regenerative dentistry. Scientists are investigating ways to harness these powerful molecules to enhance the body’s own repair mechanisms or even to regenerate dental tissues more comprehensively. This field explores using purified growth factors, gene therapy approaches to deliver growth factor genes, or smart biomaterials designed to release growth factors in a controlled manner at sites of dental injury. The ultimate, though still distant, goal of some research avenues is the regeneration of a whole tooth. While this remains a significant scientific challenge, understanding the precise sequence and combination of growth factors that orchestrate natural tooth development provides a crucial roadmap for such ambitious endeavors. Current research often focuses on regenerating components of the tooth, such as the dentin-pulp complex, by applying growth factors to stimulate resident stem cells or by combining growth factors with scaffolds and cells in tissue engineering strategies. Challenges include ensuring the correct spatial and temporal delivery of these potent molecules and avoiding unwanted side effects.
The Intricate Web: Crosstalk and Complexity
It’s crucial to understand that growth factors do not operate in isolation. Tooth development and repair are governed by a complex, interconnected network of signaling pathways. One growth factor can influence the expression or activity of another, creating intricate feedback loops and synergistic or antagonistic effects. For instance, SHH, FGF, and BMP signaling pathways are known to interact extensively during cusp patterning. The concentration gradients of these factors, their precise timing of expression, and the availability of their specific receptors all contribute to the exquisite control observed in odontogenesis. This complexity is what makes the process so robust yet also sensitive to disruptions that can lead to dental anomalies.
Concluding Thoughts on Nature’s Tiny Architects
Growth factors are undeniably the unsung heroes of tooth development, masterfully conducting every phase from initial blueprint to final structure. Their continued presence and activity are also vital for the limited repair processes our teeth can mount in response to injury. As our understanding of these molecular signals deepens, so too does the potential for developing novel biologically-based strategies in dentistry. While much research is still needed, the study of growth factors continues to illuminate the fundamental biology of our teeth and offers exciting prospects for the future of dental health, focusing on enhancing the body’s inherent regenerative capacities.