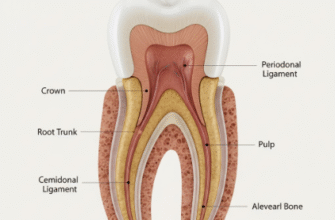
The gleaming, hard outer layer of our teeth, known as enamel, is quite a marvel of biological engineering. It’s the hardest substance in the human body, designed to withstand the immense forces of chewing and protect the sensitive inner parts of the tooth. What’s truly fascinating is how this incredibly mineralized tissue is formed, a process called amelogenesis. Unlike bone, enamel is acellular once mature, meaning it cannot repair itself. This makes its initial formation absolutely critical, and at the heart of this intricate construction are specialized proteins, with amelogenins and enamelins playing starring roles.
The Symphony of Enamel Creation
Amelogenesis is a highly orchestrated process carried out by specialized cells called ameloblasts. These cells go through a complex life cycle, first secreting the protein matrix and then facilitating its mineralization and maturation. Think of it like building a crystal palace: first, a scaffold is laid down, and then the beautiful, hard crystal structures are meticulously grown within and around it. The final step involves removing most of the scaffolding to allow the crystals to pack tightly, giving enamel its signature hardness. This entire sequence is tightly regulated, and the proteins involved are the unsung heroes determining the final architecture and properties of enamel.
The Protein Powerhouses: Amelogenins and Enamelins
While enamel is about 96% mineral by weight in its mature state, the initial organic matrix, which is predominantly protein, is indispensable for its development. This protein-rich environment doesn’t just passively fill space; it actively directs the where, when, and how of mineral crystal formation. Among the cast of enamel matrix proteins (EMPs), two stand out for their significant contributions and distinct functions: amelogenins, the most abundant, and enamelins, present in smaller quantities but equally vital.
Amelogenin: The Master Architect
Amelogenin is the dominant protein in the developing enamel matrix, making up around 90% of the total protein content during the secretory phase. Its primary job isn’t to become part of the final mineral structure, but rather to act as a sophisticated scaffolding system. These proteins are known for their ability to self-assemble into tiny spherical structures called nanospheres. These nanospheres create a three-dimensional framework that separates and organizes the nascent hydroxyapatite crystals. By doing so, amelogenins control the size and shape of these crystals, ensuring they grow into long, thin ribbons, which is characteristic of enamel’s unique prismatic structure.
Imagine a construction site where workers are carefully laying bricks. Amelogenin nanospheres act like temporary spacers and guides, ensuring each “brick” (hydroxyapatite crystal) is placed correctly and doesn’t clump together haphazardly. This controlled growth is crucial for preventing a disorganized jumble of minerals and instead fostering the highly ordered, interwoven crystal arrangement that gives enamel its incredible strength and fracture resistance. Amelogenin undergoes a series of processing steps, being cleaved by proteinases almost as soon as it is secreted. This dynamic processing is thought to modulate its interactions and prepare it for eventual complete removal during the maturation stage.
Amelogenins are the most prevalent proteins within the enamel organic matrix during its formative secretory stage. Their chief responsibility is to orchestrate the organized growth and orientation of hydroxyapatite crystals. These proteins characteristically self-assemble into nanospherical structures, thereby establishing an essential scaffold. This scaffolding is critical for preventing the premature fusion of crystals and for dictating their uniquely elongated morphology.
The gene for amelogenin, AMELX, is located on the X chromosome (with a less active homolog, AMELY, on the Y chromosome). This X-linkage has implications for certain inherited enamel defects. Furthermore, alternative splicing of the amelogenin mRNA results in several different amelogenin isoforms, each potentially having subtly different roles or functions in the enamel matrix, adding another layer of complexity and control to the formation process.
Enamelin: The Nucleation Specialist
While amelogenins are busy organizing the overall structure, enamelins, encoded by the ENAM gene, are thought to play a more direct role in the very beginning of crystal formation – the nucleation event. Although present in much smaller quantities than amelogenins (around 1-5% of the matrix proteins), enamelins are considered essential for initiating the growth of hydroxyapatite crystals at the dentin-enamel junction (DEJ) and along the secretory surface of the ameloblasts. They are believed to bind tightly to the mineral phase and may serve as templates upon which the first mineral crystals precipitate.
Enamelins are more acidic proteins compared to amelogenins and tend to be more strongly associated with the apatite crystals. Some researchers propose that enamelin molecules, or fragments thereof, might remain more persistently within the mature enamel, particularly at crystal boundaries or within crystal defects, potentially influencing the mechanical properties of the final tissue. Their interaction is not just with the mineral; it’s also suggested that enamelins might interact with the ameloblast cell membrane, helping to anchor the developing enamel layer to the cells that are producing it. This ensures a cohesive interface as the enamel layer thickens.
Though significantly less abundant than amelogenins, enamelins are widely regarded as critical for kick-starting crystal formation. They are understood to bind with high affinity to the mineral phase of enamel. Furthermore, some scientific evidence suggests they may also engage with the ameloblast cell membrane, thereby anchoring the forming enamel structure securely to the cell. This function is vital for controlled enamel apposition.
Like amelogenin, enamelin also undergoes proteolytic processing, which likely modifies its function and facilitates its role in different stages of amelogenesis. The precise mechanisms are still a subject of intense research, but their importance is underscored by the severe enamel defects that occur when the ENAM gene is mutated.
A Coordinated Dance: How They Work Together
It’s crucial to understand that amelogenins and enamelins don’t work in isolation; they are part of a finely tuned ensemble. The current understanding suggests a cooperative model. Enamelins might be among the first proteins to lay down the groundwork, initiating crystal nucleation, perhaps at specific sites along the collagen fibers of the underlying dentin or directly at the ameloblast surface. Once these initial crystal seeds are formed, amelogenins step in to control their subsequent growth and organization.
The amelogenin nanosphere scaffold provides the space and direction for these crystals to elongate into their characteristic ribbon-like morphology, preventing them from growing too wide or fusing prematurely. As mineralization progresses, other proteins like ameloblastin (also known as amelin or shealthlin) and tuftelin also play roles, though their functions are perhaps less clearly delineated than those of amelogenin and enamelin in the bulk of enamel formation. This interplay ensures that the enamel structure is not only highly mineralized but also incredibly organized from the nano-scale to the micro-scale, leading to its exceptional mechanical performance.
The Mineralization Marvel
The actual hardening of enamel involves the deposition of calcium and phosphate ions, which crystallize to form hydroxyapatite [Ca10(PO4)6(OH)2]. The protein matrix, rich in amelogenins and containing enamelins, creates a supersaturated environment with respect to these ions, but in a controlled manner. Enamelins likely provide the specific sites for heterogeneous nucleation, reducing the energy barrier for crystal formation. Once nucleated, the crystals begin to grow.
Amelogenins, through their hydrophilic and hydrophobic domains, regulate this growth. They are thought to bind to specific crystal faces, inhibiting growth in certain directions while allowing elongation in others. This results in the long, slender crystals characteristic of enamel. The spaces created by the amelogenin nanospheres are gradually filled by these growing crystals. The precise pH and ionic concentrations within the matrix, also regulated by the ameloblasts, are critical for this process to occur correctly. It’s a delicate balance of promoting mineralization while simultaneously guiding its precise form and extent.
The Great Protein Escape: Maturation
A key feature of enamel development is that the vast majority of the organic matrix, including most of the amelogenins and processed enamelin fragments, must be removed to allow the enamel to achieve its full mineralization and hardness. This occurs during the maturation stage of amelogenesis. Ameloblasts switch their function from primarily secretion to actively reabsorbing water and protein fragments, while also pumping in more mineral ions. Specific enzymes, such as matrix metalloproteinase-20 (MMP-20 or enamelysin) and kallikrein-4 (KLK4), are responsible for the progressive degradation of the enamel proteins into smaller, more easily reabsorbed pieces.
MMP-20 is active during the secretory stage, initiating the cleavage of amelogenins and other proteins. KLK4 takes over during the maturation stage, performing the final, more complete breakdown. If this protein removal process is inefficient, residual proteins can become trapped within the enamel, leading to a softer, hypomineralized tissue that is more susceptible to wear and decay. The near-complete removal of the protein scaffold allows the hydroxyapatite crystals to grow wider and pack more tightly together, resulting in the dense, extremely hard tissue that is mature enamel.
When the Blueprint Has Flaws
The critical roles of amelogenin and enamelin are starkly highlighted when their production or function is compromised. Genetic variations or mutations in the AMELX or ENAM genes can lead to a group of inherited conditions collectively known as amelogenesis imperfecta (AI). These conditions result in enamel that can be thin, soft, discolored, or easily damaged. The specific appearance and severity of the enamel defect often correlate with the particular protein affected and the nature of the genetic alteration. This underscores how dependent normal enamel formation is on the precise quantity, quality, and processing of these key structural proteins. Studying these natural “experiments” has provided invaluable insights into the specific functions of each protein in the complex choreography of amelogenesis.
In conclusion, the formation of dental enamel is a testament to biological precision, a process where amelogenins and enamelins are indispensable conductors. Amelogenins, as the principal architects, meticulously sculpt the space and guide the growth of mineral crystals, ensuring an organized and robust structure. Enamelins, though less voluminous, are crucial for initiating this mineralization, laying the very foundation upon which enamel is built. Together, along with other matrix components and cellular activity, they transform a soft protein gel into the hardest tissue in the vertebrate body. Understanding their distinct and cooperative roles not only deepens our appreciation for this biological marvel but also provides a basis for comprehending how disturbances in their function can impact dental health and structure.