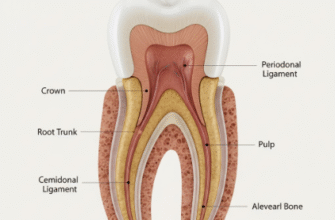
Hidden deep within the seemingly solid structure of a tooth lies a vibrant, living core known as the dental pulp. This soft connective tissue, often colloquially referred to as the “nerve” of the tooth, is far more than just a sensory apparatus. It is a complex biological system responsible for the tooth’s formation, its continued vitality, and its ability to respond to external stimuli. Understanding the pulp means understanding the life force of the tooth itself. This vitality is critically dependent on a microscopic, yet profoundly significant, circulatory system.
Like any living tissue, the dental pulp has constant metabolic demands. It requires a steady stream of oxygen and nutrients to fuel its cellular activities, including the crucial work of the odontoblasts – the specialized cells responsible for producing and maintaining dentin, the hard tissue beneath the enamel. Equally important is the efficient removal of metabolic waste products, which, if allowed to accumulate, could compromise tissue health and function. This essential life support system, ensuring both supply and sanitation, is delivered via a sophisticated and intricate network of blood vessels, with capillaries at its very core.
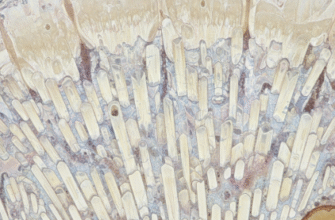
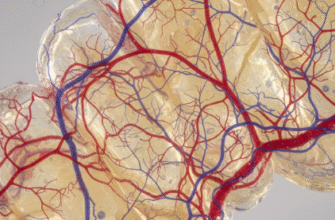
At the heart of this vascular system are the capillaries, the smallest blood vessels in the body. These microscopic conduits, often thinner than a human hair, form an incredibly dense and elaborate web throughout the entire pulp tissue. Unlike larger arteries and veins that act as major highways for blood transport, capillaries are the local streets and tiny alleyways where the crucial exchange between blood and tissue takes place. It is across their exceptionally thin walls that oxygen, vital nutrients, and various signaling molecules pass from the blood to the pulp cells, and conversely, waste products such as carbon dioxide journey in the opposite direction to be carried away.
The Architecture of Pulpal Microcirculation
The journey of blood into the dental pulp typically begins at the tooth’s apex, through one or more small openings collectively known as the apical foramen. One or several small arterioles, which are diminutive branches from larger arteries supplying the jawbones, pass through this foramen. Once inside the relatively confined space of the pulp chamber (in the crown of the tooth) and the root canals, these arterioles begin to branch extensively. This branching pattern is often described as tree-like, with progressively smaller vessels extending outwards and coronally, towards the top of the tooth, to perfuse the entire pulpal tissue.
Branching Patterns and Regional Density
As these arterioles ascend towards the crown and radiate peripherally, they give rise to an exceedingly rich network of terminal arterioles, which subsequently feed into the vast capillary beds. The distribution of these capillaries within the pulp is not uniform; rather, it is strategically organized. There is a particularly high concentration of capillaries in the peripheral region of the pulp, especially in what is known as the sub-odontoblastic zone. This zone lies just beneath the layer of odontoblasts, the cells lining the pulp cavity adjacent to the dentin. This strategic placement ensures that these metabolically active, dentin-forming cells have priority access to the blood supply. The core of the pulp, or the central pulp region, also possesses a capillary network, though it is typically less dense than that found in the periphery, particularly the coronal periphery.
The density of this capillary network is truly remarkable. In the coronal pulp, especially near the odontoblasts, the capillaries form such a dense meshwork that nearly every cell is in close proximity to a blood source. This proximity minimizes diffusion distances, allowing for highly efficient exchange of substances. This dense vascularization is a hallmark of tissues with high metabolic rates, and the dental pulp, with its continuous dentinogenic and sensory activities, certainly qualifies.
The dental pulp exhibits an exceptionally rich capillary bed, particularly concentrated in the coronal pulp and directly beneath the odontoblastic layer. This high degree of vascularization directly reflects the significant metabolic activity of the pulp, especially related to ongoing dentinogenesis and the maintenance of sensory functions. The intricate three-dimensional arrangement of these capillaries ensures that almost every pulpal cell is positioned relatively close to a blood supply, facilitating rapid and efficient nutrient exchange and waste removal, which are crucial for sustained pulp vitality.
Structure of Pulp Capillaries
Pulpal capillaries are fine-bore tubes, often just wide enough for red blood cells to pass through in single file, a process that maximizes their surface area contact with the capillary wall for exchange. Their walls are extremely thin, primarily composed of a single layer of flattened endothelial cells. These endothelial cells are joined together by cell junctions and are surrounded by a supportive basement membrane and, occasionally, by cells called pericytes. Pericytes are contractile cells that can influence capillary blood flow and permeability. This delicate, minimalistic structure is perfectly optimized for the primary function of capillaries: efficient exchange of gases, nutrients, and waste products between the blood and the surrounding interstitial fluid of the pulp.
Vital Functions Supported by the Capillary Network
The intricate capillary network within the dental pulp underpins several critical functions essential for the tooth’s health and longevity.
Nourishment and Respiration for Pulpal Cells
The foremost role of the pulpal capillaries is to sustain the life of all the cells residing within the pulp. This includes odontoblasts, fibroblasts (which produce the connective tissue matrix), undifferentiated mesenchymal cells, immune cells, and nerve cells. All these cellular components depend on the constant delivery of glucose for energy, amino acids for protein synthesis, and, crucially, oxygen for cellular respiration. Odontoblasts, in particular, have consistently high energy demands. This is due to their continuous role in synthesizing and mineralizing dentin throughout the life of the tooth. This includes the formation of primary dentin during tooth development, secondary dentin throughout life which gradually reduces pulp volume, and tertiary or reparative dentin in response to minor stimuli or wear.
Efficient Waste Product Evacuation
Cellular metabolism, while essential for life, inevitably produces waste products. These include carbon dioxide, lactic acid, and other metabolic byproducts. If these substances were allowed to accumulate within the confined space of the pulp chamber, they would create a toxic environment, impairing cellular function and potentially leading to irreversible damage. The capillary network, working in concert with venules (small veins), efficiently collects these waste products from the pulpal interstitial fluid. These are then transported out of the pulp via the venous drainage system, which largely mirrors the arteriolar entry, exiting through the apical foramen. This continuous cleansing process is vital for maintaining a healthy, stable pulpal environment.
Supporting Sensory and Defensive Roles
While the numerous nerve fibers within the pulp are responsible for transmitting sensory information (such as sensitivity to temperature changes or pressure), their ability to function optimally and respond accurately is dependent on a healthy, well-nourished, and stable environment. The capillary network ensures this by providing the necessary metabolic support to these neural tissues. Furthermore, the blood vessels, including capillaries, play a crucial role in the pulp’s innate defense mechanisms. In response to mild irritation or minor injury, the local vasculature can respond dynamically. Blood flow can increase (hyperemia), delivering immune cells, antibodies, and various inflammatory mediators from the bloodstream to the affected site. This vascular response is a key part of the pulp’s ability to protect itself, neutralize threats, and initiate repair processes, highlighting the dynamic and responsive nature of this microcirculatory system.
Maintaining Pulp Hydration and Intrinsic Pressure
The exchange of fluid across capillary walls, governed by a balance of hydrostatic and osmotic pressures, also contributes significantly to maintaining the appropriate hydration levels and interstitial fluid pressure within the pulp. This is particularly important because the dental pulp is uniquely situated: it is enclosed within rigid, unyielding dentin walls. This anatomical confinement means the pulp has very limited capacity to expand if fluid accumulates. The microcirculation, through its regulation of fluid exchange, plays a critical role in managing this delicate pressure balance, preventing excessive fluid buildup that could compress cells and blood vessels, thereby compromising pulpal health.
Dynamics and Regulation of Pulpal Blood Flow
Blood flow through the pulpal capillary network is not a static, unchanging phenomenon. Instead, it is dynamically regulated to meet the ever-changing metabolic needs of the tissue and to respond to various stimuli.
Intrinsic Control Mechanisms
The regulation of pulpal blood flow involves a complex interplay of local factors, neural influences, and even systemic circulatory conditions. At the local level, smooth muscle cells present in the walls of arterioles and precapillary sphincters can contract (vasoconstriction) or relax (vasodilation). These actions alter the diameter of these resistance vessels, thereby increasing or decreasing the volume of blood entering the downstream capillary beds. Various local chemical mediators, often released by pulpal cells themselves (like odontoblasts or endothelial cells) in response to changes in oxygen levels, pH, or metabolic activity, can also directly influence vessel diameter. For instance, a buildup of metabolic byproducts like adenosine or carbon dioxide can trigger vasodilation to increase blood flow and clear these substances.
Neural Influences on Vasomotor Tone
The dental pulp is richly innervated, and a significant portion of this innervation includes autonomic nerve fibers, particularly sympathetic nerves, which are closely associated with the pulpal blood vessels. Activation of these sympathetic nerves typically releases norepinephrine, which causes vasoconstriction, thereby reducing blood flow. Conversely, certain neuropeptides released from sensory nerve fibers (which are primarily responsible for pain transmission) can have a vasodilatory effect. This complex neurovascular interaction allows for rapid adjustments in pulpal blood flow in response to both local needs and broader physiological signals.
Age-Related Changes in Pulpal Vasculature
Like many tissues in the human body, the dental pulp undergoes discernible changes with advancing age. There is often a gradual reduction in the overall volume of the pulp chamber and root canals. This reduction is primarily due to the continuous, lifelong deposition of secondary dentin on the inner walls of the pulp cavity. Concurrently with this decrease in pulp space, there can also be a slight, progressive decrease in the overall vascularity and cellularity of the pulp tissue. Fibrotic changes may also become more apparent. However, even in older teeth, a functional and responsive capillary network remains absolutely essential for maintaining the vitality and sensory capabilities of the pulp. The adaptive capacity might be somewhat reduced, but the fundamental reliance on this microcirculation persists.
The Unseen Guardian of Tooth Vitality
The intricate, almost invisibly fine network of capillaries within the dental pulp is a true marvel of biological engineering and efficiency. Though completely hidden from view beneath layers of enamel and dentin, this microcirculatory system is the unsung hero, tirelessly working to maintain the tooth’s vitality. It is responsible for its ability to form and repair dentin, its capacity to sense and respond to its environment, and its overall biological integrity. The sheer efficiency of this network in delivering life-sustaining substances and removing potentially harmful waste products is fundamental to the long-term health and function of every single tooth.
Any significant disruption to this delicate vascular network, whether through trauma, extensive decay, or other insults, can have profound consequences for the health of the pulp and, ultimately, for the survival of the tooth as a living organ. Appreciating the complexity and critical importance of this pulpal capillary system deepens our understanding of the tooth not merely as a hard, inert structure, but as a living, dynamic, and responsive biological entity. The constant, silent, and meticulous work performed by these myriad tiny vessels underscores the delicate physiological balance required to keep our teeth healthy, functional, and vital throughout our lives. This hidden circulatory system is, in essence, the lifeline of the tooth.