Often taken for granted, that watery fluid in our mouths does far more than just moisten food. Saliva is a complex biological liquid, a true multitasker that plays a pivotal role in maintaining our oral health and even our overall well-being. While it aids in digestion, helps us speak, and allows us to taste the myriad flavors of our food, one of its most crucial, yet perhaps least celebrated, functions is its ability to act as a powerful natural defender against acids. This protective mechanism is constantly at work, safeguarding our teeth from the daily onslaught of acidic challenges.
The Acidic Assault on Our Oral Environment
Our mouths are frequently exposed to acids. These do not just magically appear; they come from two primary sources. Firstly, many foods and drinks we consume are inherently acidic. Think of citrus fruits like lemons and oranges, fizzy sodas, sports drinks, wine, and even some salad dressings. When these substances enter the mouth, they directly lower the pH, creating an acidic environment.
Secondly, and perhaps more insidiously, acids are produced by bacteria residing in our mouths. These microorganisms thrive on sugars and fermentable carbohydrates found in our diet – sweets, bread, pasta, and sugary snacks are prime examples. As bacteria metabolize these sugars, they release acidic byproducts, primarily lactic acid. This process is what is commonly referred to as an “acid attack.”
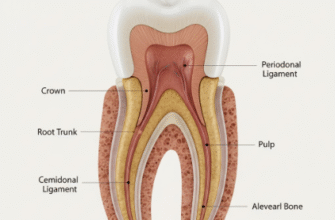
The pH scale, ranging from 0 (highly acidic) to 14 (highly alkaline), with 7 being neutral, helps us understand this. Healthy saliva typically maintains the oral pH around 6.2 to 7.6. However, when acids are introduced or produced, this pH can plummet. Tooth enamel, the hard, protective outer layer of our teeth, begins to demineralize or dissolve when the pH drops below a critical level, generally around 5.5. Repeated or prolonged exposure to these acidic conditions can lead to dental erosion and the formation of cavities.
Saliva to the Rescue: A Natural Neutralizing Champion
Fortunately, our bodies have an inbuilt defense mechanism against these acid attacks: saliva. It employs several sophisticated strategies to counteract acidity and restore a neutral pH in the mouth. This neutralizing capacity is largely due to its remarkable chemical composition and physical properties.
The Buffering Brigade: Chemical Warfare Against Acidity
Saliva contains several buffering systems. A buffer is a solution that can resist changes in pH upon the addition of an acidic or basic component. It is like a chemical shock absorber for acidity.
Bicarbonate (HCO3-): The Primary Defender. This is the most important buffering system in saliva. Bicarbonate ions are alkaline and readily react with hydrogen ions (H+), which are responsible for acidity. The reaction looks something like this: H+ (acid) + HCO3- (bicarbonate) → H2CO3 (carbonic acid). Carbonic acid is a weak acid and can further break down into water (H2O) and carbon dioxide (CO2), which can then be exhaled or swallowed. This effectively mops up the excess acid, raising the pH back towards neutral.
Phosphate System: A Steadfast Ally. While not as potent as the bicarbonate system, various forms of phosphate ions present in saliva also contribute to buffering. These phosphate compounds can accept or release hydrogen ions as needed to help stabilize the oral pH. This system works in concert with bicarbonate, providing an additional layer of acid-neutralizing defense.
Proteins and Peptides: The Unsung Buffers. Saliva is rich in various proteins and peptides, some of which possess buffering capabilities. For example, a peptide called sialin can help to rapidly raise the pH in dental plaque after exposure to sugar. Other proteins have amino acid side chains that can accept or donate protons, contributing to the overall buffering capacity of saliva.
Urea’s Unexpected Contribution. Saliva contains urea, a waste product. Certain oral bacteria can break down urea into ammonia and carbon dioxide. Ammonia is alkaline and can help neutralize acids, thereby increasing the pH in dental plaque. This is another fascinating, indirect way saliva combats acidity.
Saliva’s buffering system, primarily driven by bicarbonate, begins to neutralize acids produced by bacteria within minutes of a sugar-induced acid attack. This rapid response is crucial for protecting tooth enamel from demineralization. Without this efficient natural defense, our teeth would be significantly more susceptible to decay and erosion, highlighting saliva’s indispensable role in maintaining oral health.
The Power of Flow and Clearance: Physical Defenses
Beyond its chemical prowess, saliva’s physical properties are also vital in managing oral acidity.
Salivary Flow Rate: Washing It Away. The sheer volume of saliva produced and its flow rate play a significant role. When we eat, or even think about food, salivary flow increases dramatically (this is called stimulated saliva). This increased flow helps in several ways:
- It dilutes acids, reducing their concentration and erosive potential.
- It physically washes away food particles and sugars that bacteria would otherwise feed on to produce more acid.
- It delivers more buffering components, like bicarbonate, to the sites where they are needed most.
Clearance Action: Sweeping the Decks. Saliva does not just dilute; it actively helps clear substances from the mouth. The constant swallowing reflex, aided by saliva’s lubricating properties, ensures that acids and food debris do not linger in the oral cavity for too long. The faster these harmful substances are cleared, the less time they have to damage teeth.
When Saliva’s Defenses are Overwhelmed
While saliva is remarkably effective, its neutralizing capacity is not infinite. If acid attacks are too frequent or too strong, or if saliva production is compromised, problems can arise. Consuming sugary or acidic foods and drinks very frequently throughout the day means saliva is constantly battling to restore neutral pH, and it may not always win. This continuous assault can overwhelm its buffering capacity, leading to net mineral loss from teeth.
A condition known as xerostomia, or dry mouth, significantly impairs saliva’s protective functions. Reduced salivary flow, which can result from various factors such as side effects of some medications, certain systemic health conditions, particular medical treatments, or simply not drinking enough water, means there is less dilution of acids and fewer buffering agents available. This situation can dramatically increase the risk of cavities and dental erosion.
Beyond Neutralization: Saliva’s Other Supportive Roles
While acid neutralization is a key protective function, saliva also helps maintain oral health in other ways that indirectly support this goal. For instance, saliva is supersaturated with calcium and phosphate ions. After an acid attack has caused some initial demineralization, these minerals in saliva can help to remineralize or repair the early damage to tooth enamel, provided the pH is restored to a more neutral level. It also contains antimicrobial components like lysozyme and lactoferrin that help control bacterial populations, thereby indirectly reducing acid production.
Nurturing Your Natural Oral Guardian
Given the vital role saliva plays, it is sensible to support its function. Simple lifestyle measures can make a big difference:
- Stay Hydrated: Drinking plenty of water throughout the day is essential for maintaining adequate salivary flow. Water itself also helps rinse the mouth.
- Chew Sugar-Free Gum: Chewing, especially gum containing xylitol, stimulates saliva production significantly, enhancing its acid-neutralizing and clearing effects.
- Mind Your Diet: Limit the frequency of consuming sugary and acidic foods and drinks. If you do indulge, try to have them with meals rather than sipping or snacking on them throughout the day.
- Practice Good Oral Hygiene: Regular brushing and flossing remove plaque and food debris, reducing the fuel available for acid-producing bacteria.
In conclusion, saliva is far more than just mouth water. It is a sophisticated, dynamic fluid with an incredibly important job: protecting our teeth and oral tissues from the constant threat of acid damage. Its buffering systems, flow rate, and clearance mechanisms work in concert to neutralize harmful acids, wash away debris, and even help repair early tooth damage. Understanding and appreciating the importance of saliva in neutralizing acids can motivate us to adopt habits that support this unsung hero of our oral health, ensuring it can continue its vital work effectively.