Ponder for a moment the incredible structures within your own body. Among them, a remarkable material stands out, silently performing a vital role every single day. We’re talking about tooth enamel, the glistening, hard outer layer of your teeth. It’s more than just a pretty surface; it’s a feat of biological engineering, a shield that guards the sensitive inner parts of your teeth from a daily barrage of challenges, from the crunch of an apple to the chill of ice cream. This unsung hero deserves a closer look, for its story is one of strength, resilience, and fascinating complexity.
What Exactly is This Super Shield?
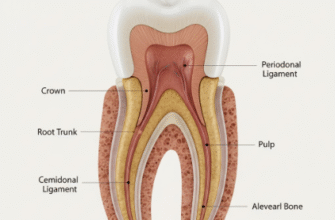
So, what makes up this extraordinary material? Tooth enamel is primarily composed of an incredibly dense mineral called hydroxyapatite, which is a crystalline calcium phosphate. Imagine microscopic crystals packed together with astonishing precision, forming a structure that is, in fact, the hardest substance in the entire human body. It’s even tougher than bone! This hardness is precisely what allows our teeth to withstand the significant forces of biting and chewing over a lifetime. Its mineral nature also gives it a characteristic translucency, meaning light can pass partially through it. The color of your teeth is actually a combination of this translucent enamel and the color of the dentin layer beneath it.
The Making of a Masterpiece: Enamel Formation
The creation of enamel, a process known as amelogenesis, is a carefully orchestrated biological event that occurs even before a tooth makes its grand entrance into the mouth. Specialized cells called ameloblasts are the master artisans behind this. These cells work diligently, laying down the enamel matrix layer by layer, gradually mineralizing it to achieve its incredible hardness. Think of it like tiny builders constructing an intricate, super-strong wall. Once their job is done and the tooth has fully formed its enamel crown, these ameloblast cells disappear. This is a crucial point: because they don’t stick around, the body cannot create new enamel later in life if the original layer is damaged or wears away.
A Non-Living Guardian
Because the ameloblasts vanish after enamel formation, the enamel itself is not a living tissue. It contains no nerves or blood vessels, which is why you don’t feel pain from the enamel surface itself. However, this also means it lacks the ability to regenerate or repair itself in the way that, say, skin heals after a cut or bone mends after a fracture. Once it’s gone, it’s gone for good, making its preservation absolutely paramount for long-term dental health. This non-living status underscores its role as a passive, yet incredibly robust, protective layer.
Why Enamel Reigns Supreme (Its Functions)
The primary role of enamel is, without a doubt, protection. It acts as a durable barrier, shielding the softer, more sensitive underlying dentin and pulp from a multitude of daily assaults. Firstly, it provides the necessary strength to withstand the mechanical stresses of mastication – all the biting, tearing, and grinding of food. Without enamel, our teeth would quickly wear down. Secondly, it insulates the tooth from extreme temperatures. That searing hot coffee or freezing cold ice cream would be agony without enamel buffering the sensitive nerves within. Lastly, it offers a line of defense against chemical attacks, particularly from acids found in our diet or produced by bacteria in the mouth.
The Enemies at the Gates: Threats to Enamel Integrity
Despite its remarkable strength, enamel is not invincible. It faces several adversaries that can gradually weaken and erode it. Perhaps the most pervasive and insidious of these is acid attack – the silent saboteur. Acids can come from two main sources. Dietary acids are found in many common foods and drinks like citrus fruits (lemons, oranges), sodas, sports drinks, wine, and even some fruit juices. When these acids frequently wash over the teeth, they can slowly dissolve the mineral structure of the enamel. The other major source is bacterial acids. Bacteria in our mouths feed on sugars and starches from our food, and as a byproduct of their metabolism, they produce acids. These acids, if left unchecked, can demineralize enamel, leading to the formation of cavities.
Beyond chemical warfare, enamel also contends with physical foes – wear and tear that contribute to its gradual loss. Abrasion is one such enemy, often caused by brushing too aggressively, using a hard-bristled toothbrush, or employing highly abrasive toothpastes. This mechanical wearing can scrub away enamel over time. Then there’s attrition, which is the wear that occurs from tooth-on-tooth contact. This is commonly seen in individuals who clench or grind their teeth (a condition known as bruxism), often unknowingly during sleep. Finally, erosion is the broader term for the chemical loss of tooth mineral not caused by bacteria, predominantly from acidic foods, drinks, or even stomach acids in cases of acid reflux.
Once mature enamel is lost, the body cannot naturally regenerate it, making proactive protection absolutely crucial. This irreversible loss can lead to increased tooth sensitivity and a higher susceptibility to cavities. Be particularly mindful of frequent consumption of acidic foods and beverages, as these are primary culprits in wearing down this vital protective layer of your teeth. Protecting what you have is key to long-term oral comfort and health.
Can We Mend a Broken Shield? Remineralization and Beyond
While significant enamel loss is permanent, there’s a fascinating natural process at play for minor, early-stage demineralization. Our mouths are in a constant state of flux, with minerals being lost (demineralization) and regained (remineralization). Saliva plays a heroic role here. It’s not just water; it’s rich in calcium and phosphate ions, the very building blocks of enamel. Saliva helps to neutralize acids in the mouth and constantly bathes the teeth in these minerals, allowing for a degree of natural repair. Fluoride, a mineral commonly found in toothpaste and sometimes in drinking water, significantly enhances this remineralization process. It can integrate into the enamel structure, forming fluorapatite, which is even more resistant to acid attacks than the original hydroxyapatite. However, it’s important to understand that remineralization can only help with the very initial stages of enamel weakening, not rebuild substantial lost structure.
Fortifying Your Defenses: Protecting Your Priceless Enamel
Given that enamel cannot regrow, prevention is undoubtedly the best strategy. Protecting this precious layer involves a combination of mindful habits. Dietary diligence is a cornerstone. This means being aware of and limiting the consumption of highly sugary and acidic foods and drinks. When you do indulge, try to consume them with meals rather than sipping or snacking on them throughout the day, which prolongs acid exposure. Rinsing your mouth with plain water after consuming acidic items can help neutralize acids. Including calcium-rich foods in your diet, like dairy products or leafy greens, also supports overall dental health by providing the minerals needed for strong teeth and bones, and for saliva to do its remineralizing work.
Alongside a smart diet, oral hygiene habits are critical. This starts with proper brushing technique. It’s not about scrubbing hard, but about cleaning effectively. Use a soft-bristled toothbrush and gentle, circular motions, ensuring you reach all surfaces of your teeth. Most dental professionals recommend brushing twice a day. The choice of toothpaste matters too; using a fluoride toothpaste is highly beneficial as it aids in the remineralization process and strengthens enamel against acid attacks. Don’t forget that regular dental check-ups and cleanings allow professionals to spot early signs of enamel wear and provide guidance. And a simple but often overlooked tip: avoid using your teeth as tools to open packages or bite non-food items, as this can chip or crack your valuable enamel.
Enamel’s Unique Quirks and Wonders
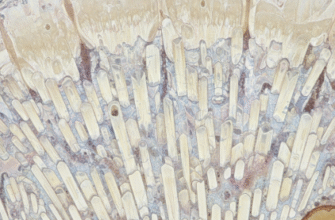
Delving deeper into enamel’s structure reveals even more of its marvels. It’s not just a solid block; it’s composed of millions of microscopic enamel rods, or prisms, that run from the surface of the enamel towards the underlying dentin. These rods are packed incredibly tightly in an intricate, keyhole-like pattern, contributing to enamel’s strength and fracture resistance. The thickness of enamel also isn’t uniform across the entire tooth. It’s thickest at the cusps – the biting surfaces – where the most force is exerted, and thinnest near the gumline. The overall integrity of your enamel directly impacts not just the health of individual teeth but your overall oral comfort and function, influencing everything from what you can comfortably eat to the brightness of your smile.
Tooth enamel truly is a remarkable substance, a natural shield forged with incredible strength and precision. Understanding its unique properties, how it’s formed, what threatens it, and how to protect it empowers us to take better care of this invaluable asset. While it may not be alive, its presence is vital for a healthy, functioning, and comfortable smile throughout our lives. Cherishing and safeguarding your enamel is an investment that pays daily dividends in oral well-being.