The emergence of a tooth into the oral cavity, a process commonly known as tooth eruption, is far more intricate than a simple upward push. It’s a meticulously choreographed ballet of cellular activities and molecular signals, ensuring the tooth navigates from its developmental crypt within the jawbone to its final, functional position. Understanding this journey requires delving into the dynamic world of specialized cells and the complex communications that govern their behavior, orchestrating the precise resorption and formation of bone, and the development of supportive tissues.
The Dental Follicle: The Eruptive Engine’s Core
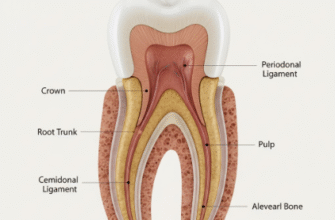
At the heart of tooth eruption lies the dental follicle, a sac of connective tissue derived from ectomesenchyme that envelops the developing tooth crown before root formation begins. This structure is not merely a passive wrapper; it’s the primary engine driving many eruptive events. Cells within the dental follicle, often referred to as dental follicle cells (DFCs), are a heterogeneous population with remarkable capabilities. They are the master regulators, orchestrating the necessary bone resorption to create an eruption pathway and guiding the formation of the tooth’s attachment apparatus, the periodontium.
DFCs respond to signals from the tooth itself, particularly from the stellate reticulum and the reduced enamel epithelium (REE) overlying the crown. One critical signal is Parathyroid Hormone-related Protein (PTHrP), which stimulates DFCs to express factors that control bone-resorbing cells. These versatile DFCs can differentiate into various cell types, including osteoblasts (bone-forming cells), cementoblasts (cementum-forming cells on the root surface), and periodontal ligament fibroblasts. This differentiation potential is crucial for both carving the path and building the future support system of the tooth.
The dental follicle is indispensable for tooth eruption. Experimental removal of the dental follicle invariably halts the eruption process. This underscores its central role in coordinating the cellular machinery required for tooth movement through bone.
Bone Remodeling: The Pathway and Socket Formation
Tooth eruption fundamentally relies on bone remodeling – the coupled processes of bone resorption (breakdown) and bone formation, meticulously coordinated around the moving tooth. For a tooth to navigate from its bony crypt to the oral cavity, a precise sequence of bone destruction and construction must occur, guided by intricate cellular communication primarily orchestrated by the dental follicle.
Osteoclasts: Excavators of the Eruption Route
To move through the jawbone, a path must be cleared coronally to the advancing tooth. This critical task is performed by osteoclasts, large, multinucleated cells specialized in dissolving bone mineral and matrix. Dental follicle cells (DFCs) are pivotal in recruiting and activating these bone-resorbing giants. They achieve this by producing key signaling molecules, most notably Receptor Activator of Nuclear factor Kappa-B Ligand (RANKL) and Macrophage Colony-Stimulating Factor (M-CSF). RANKL binds to its receptor, RANK, found on osteoclast precursors (monocyte/macrophage lineage cells), stimulating their differentiation, fusion into mature osteoclasts, and subsequent activation. These activated osteoclasts then attach firmly to the bone surface overlying the tooth crown and create resorption lacunae, also known as Howship’s lacunae, effectively excavating the eruption pathway piece by piece.
The intense activity of osteoclasts is not unchecked; it is exquisitely regulated to ensure resorption occurs only where needed. DFCs also produce Osteoprotegerin (OPG), a soluble decoy receptor that avidly binds to RANKL, thereby preventing RANKL from interacting with RANK on osteoclast precursors. The dynamic balance between the pro-resorptive RANKL and the anti-resorptive OPG, often expressed as the RANKL/OPG ratio, is a critical molecular checkpoint. An increase in this ratio, favoring RANKL availability, tips the balance towards bone resorption, a condition essential for initiating and sustaining tooth eruption.
Osteoblasts: Architects of the Alveolar Socket
While osteoclasts are busy clearing the way, osteoblasts, the bone-forming cells, are concurrently, or in a precisely timed sequence, active. Their primary role during eruption is not necessarily to “push” the tooth out by depositing bone at the base of the crypt, as once widely believed, although some apical bone apposition does occur and might contribute to minor movements or stabilization. Instead, osteoblasts are crucial for remodeling and reshaping the alveolar socket as the tooth moves. They deposit new bone on the sides and at the base of the developing socket, adapting its contours to the emerging root and ensuring the continued integrity and strength of the jawbone. This coordinated bone apposition is also influenced by signals emanating from the dental follicle and mechanical cues from the moving tooth, ensuring that bone formation is spatially and temporally harmonized with the resorptive processes.
Periodontal Ligament Fibroblasts: Generating Force and Attachment
As the tooth erupts and root formation progresses, the dental follicle gives rise to the periodontal ligament (PDL), a specialized connective tissue that anchors the tooth to the alveolar bone. PDL fibroblasts are the principal cells of this ligament. These cells are not static; they are highly dynamic, responsible for synthesizing and remodeling the collagen fibers and extracellular matrix components of the PDL. There’s compelling evidence that PDL fibroblasts contribute actively to tooth movement. They possess contractile properties, attributed to actin-myosin cytoskeletal elements, enabling them to generate cellular forces. It’s hypothesized that the coordinated contraction of these fibroblasts, coupled with the organized arrangement and continuous remodeling of collagen fibers, could generate tractional forces that assist in pulling the tooth towards the oral cavity.
Furthermore, PDL fibroblasts are deeply involved in rapid matrix turnover, a process essential for allowing tooth movement while simultaneously maintaining secure attachment. They secrete matrix metalloproteinases (MMPs) that degrade old or misaligned collagen fibers and other matrix components, and concurrently synthesize new ones. This constant remodeling allows the PDL to adapt dynamically to the tooth’s changing position during its eruptive journey and later during subtle functional movements. The intricate, basket-like network of collagen fibers, constantly being reorganized by PDL fibroblasts, creates a supportive yet adaptable sling for the erupting tooth, cushioning it against masticatory forces once in function.
PDL fibroblasts are pivotal for the later stages of tooth eruption and for subsequently maintaining the tooth’s functional position. Their inherent ability to generate contractile forces and dynamically remodel the surrounding extracellular matrix is widely considered a significant contributor to the overall eruptive mechanism. This role is especially pronounced once the tooth has breached the oral mucosa and requires precise guidance into occlusion.
Molecular Conversations: The Signaling Networks Guiding Eruption
The entire sophisticated process of tooth eruption is governed by a complex interplay of signaling molecules, forming an intricate molecular conversation between various cell types. We’ve touched upon the Parathyroid Hormone-related Protein (PTHrP)-RANKL-OPG axis, which is undeniably central to regulating osteoclast activity and thus bone resorption. PTHrP, secreted by cells of the enamel organ (specifically the stellate reticulum and later the reduced enamel epithelium), acts as a key upstream initiator. It diffuses to the dental follicle, stimulating DFCs to upregulate the expression of RANKL and concomitantly downregulate the expression of its inhibitor, OPG. This critical shift in the RANKL/OPG ratio robustly promotes osteoclast formation and activity, thereby clearing the bony path for the advancing tooth.
However, this axis does not operate in isolation. Numerous other signaling molecules also play vital roles in this developmental cascade:
- Transforming Growth Factor-beta (TGF-β) family: These pleiotropic factors are involved in various aspects of eruption, including stimulating DFC proliferation and differentiation, influencing extracellular matrix production by fibroblasts, and modulating immune responses.
- Bone Morphogenetic Proteins (BMPs): Well-known for their potent role in bone and cartilage formation, BMPs also influence DFC activity and osteoblast differentiation, contributing significantly to the bone remodeling processes that shape the alveolar socket around the erupting tooth.
- Colony-Stimulating Factors (CSFs): M-CSF (Macrophage Colony-Stimulating Factor), as previously mentioned, is crucial for the survival, proliferation, and differentiation of osteoclast precursors, working synergistically with RANKL.
- Chemokines: Specific molecules like Monocyte Chemoattractant Protein-1 (MCP-1/CCL2), produced by DFCs in response to various stimuli, act as powerful attractants for monocytes (which are osteoclast precursors), recruiting them to the specific sites where bone resorption is required.
The precise spatiotemporal control of these signals is paramount for successful eruption. For instance, signals promoting bone resorption must be highly concentrated coronally to the tooth to form the eruption pathway, while signals fostering bone formation or PDL development need to be more active apically and laterally to the developing root.
Disruptions in these delicate signaling pathways can lead to significant clinical problems, such as eruption failure or ectopic eruption where teeth emerge in abnormal locations. Genetic mutations affecting key genes like those encoding RANKL, RANK, OPG, or PTHrP can result in severe tooth eruption disorders in both humans and animal models. These observations powerfully highlight the delicate molecular balance required for the normal, timely emergence of teeth.
Epithelial Contributions: The Reduced Enamel Epithelium’s Role
The Reduced Enamel Epithelium (REE) is a thin epithelial layer formed by the fusion and regression of the outer enamel epithelium and the inner enamel epithelium (ameloblasts) after enamel formation is fully complete. This epithelial layer intimately covers the unerupted tooth crown and plays a surprisingly active, multifaceted role in the eruption process. As highlighted earlier, it serves as an important source of PTHrP, thereby influencing the dental follicle. Additionally, the REE secretes various enzymes, including certain matrix metalloproteinases (MMPs) and other proteases. These enzymes are thought to help break down the remaining connective tissue matrix separating the advancing tooth from the overlying oral epithelium, thus facilitating the crucial fusion of these two epithelial layers. This fusion event is critical as it creates a continuous, epithelial-lined pathway for the tooth to emerge into the oral cavity without causing significant bleeding or inviting infection. Once the tooth tip pierces the oral mucosa, the REE contributes to the formation of the initial junctional epithelium, which forms a vital seal around the gingival cuff of the newly erupted tooth.
The Eruptive Journey: Distinct Phases of Cellular Activity
Tooth eruption is not a single, monolithic event but rather a protracted journey that can be broadly categorized into distinct phases, each characterized by a unique profile of cellular activities and movements:
- Pre-eruptive Phase: This initial phase encompasses all the developmental movements of the tooth germ within the jawbone *before* root formation begins. During this time, the dental follicle is actively differentiating, and slow, localized bone remodeling occurs to accommodate the increasing size of the growing tooth crown. Cellular activity is primarily focused on the growth and cytodifferentiation of the various tooth structures.
- Eruptive (Prefunctional) Phase: This is the main, active phase where the tooth undertakes its significant journey from its developmental position deep within the bone towards the oral cavity, continuing until it reaches functional occlusal contact with its antagonist tooth (or teeth) in the opposing jaw. This phase is hallmarked by:
- Intense and highly localized osteoclast activity in the bone coronal to the tooth, driven by DFCs under the influence of PTHrP and other pro-resorptive signals, meticulously creating the eruption pathway.
- Root formation commences and proceeds throughout this phase, though it is generally no longer considered the primary engine for the extensive eruptive movement, but rather a coordinating factor.
- Progressive development of the periodontal ligament from the dental follicle, with PDL fibroblasts actively synthesizing and organizing collagen fibers, and potentially generating important tractional forces.
- Significant bone apposition by osteoblasts apically and laterally to the moving tooth, remodeling the alveolar socket to conform to the developing root.
- The REE fuses with the oral epithelium, allowing the tooth crown to painlessly and aseptically emerge into the oral environment.
- Post-eruptive (Functional) Phase: This phase commences once the tooth has reached occlusal contact and continues, in essence, throughout the functional life of the tooth. Movements during this phase are generally much slower and more subtle. They serve primarily to compensate for occlusal and interproximal wear of the tooth surfaces and to adapt to ongoing, albeit slow, growth and remodeling of the jaws. PDL fibroblasts are the key cellular players here, mediating these fine adjustments through continuous, low-grade remodeling of the ligament. Osteoblasts and osteoclasts also remain active at a basal level to maintain the integrity and health of the surrounding alveolar bone.
In conclusion, tooth eruption stands as a marvel of biological engineering, a complex developmental process driven by a sophisticated and highly regulated cascade of cellular events. From the dental follicle’s critical orchestration of bone resorption and formation to the dynamic contractility and remodeling prowess of periodontal ligament fibroblasts, each participating cell type plays a specific, exquisitely coordinated role. The intricate network of molecular signals exchanged between these cells and tissues ensures that each tooth successfully navigates its complex journey from its hidden crypt into the oral cavity, ultimately ready to perform its vital functions in mastication and speech. While many fundamental aspects are now well understood, ongoing research continues to unveil further subtleties and molecular intricacies of this fundamental, yet still partly enigmatic, developmental process.