Ever wondered about the gleaming, hard surface of your teeth that meets the world every time you smile, speak, or chew? That impressive shield is your tooth enamel, a remarkable biological material with a fascinating story. It’s more than just a pretty facade; it’s a sophisticated, highly mineralized layer that plays a crucial role in your oral health. Understanding its basic anatomy can help you appreciate just how vital it is and why protecting it is paramount.
What Exactly is Tooth Enamel?
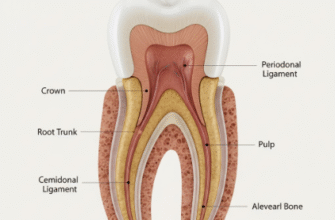
Tooth enamel is the outermost layer of the crown of your tooth – the part visible above the gum line. Think of it as the tooth’s personal armor. It’s the hardest substance in the human body, even tougher than bone! This incredible strength is essential, considering the daily wear and tear our teeth endure from biting, chewing, and even changes in temperature from the foods and drinks we consume. Its primary job is to protect the softer, more sensitive inner parts of the tooth, namely the dentin and the pulp, from damage and decay.
While it’s incredibly tough, enamel is also somewhat brittle, which is why it’s supported by the more flexible dentin underneath. It’s translucent, meaning light can pass through it, and its color can range from light yellow to grayish-white. The perceived color of your teeth is actually a combination of the enamel’s translucency and the color of the dentin beneath it.
The Building Blocks of Strength: Enamel’s Composition
The remarkable properties of enamel come down to its unique composition. It is overwhelmingly mineral – about 96% by weight. The remaining portion consists of a small amount of organic material and water.
Hydroxyapatite: The Star Mineral
The primary mineral component is a crystalline calcium phosphate called hydroxyapatite (Ca10(PO4)6(OH)2). This is the same mineral found in bone, but in enamel, the crystals are much larger and more densely packed, contributing to its superior hardness. These hydroxyapatite crystals are incredibly organized, forming the fundamental structural units of enamel.
Other minerals like fluoride, carbonate, magnesium, and sodium can also be incorporated into the hydroxyapatite crystal lattice in trace amounts. Fluoride, in particular, plays a significant role: when it replaces the hydroxyl group in hydroxyapatite, it forms fluorapatite. Fluorapatite is even more resistant to acid demineralization than hydroxyapatite, which is why fluoride is so beneficial for dental health.
Microscopic Architecture: A Closer Look at Enamel Structure
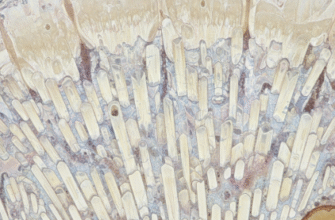
If you were to look at enamel under a powerful microscope, you wouldn’t see a simple, uniform sheet. Instead, you’d find an intricate and highly organized structure composed of millions of tiny rods or prisms, known as enamel rods or enamel prisms. These are the basic structural units of enamel.
Each enamel rod is a tightly packed bundle of hydroxyapatite crystals. These rods are long and run roughly perpendicular from the dentinoenamel junction (DEJ), the boundary between enamel and dentin, towards the tooth surface. However, they don’t always run in perfectly straight lines; they can weave and interlock, particularly near the DEJ and the cusps of teeth. This interwoven arrangement, known as gnarled enamel, adds to the enamel’s strength and resistance to fracture.
The enamel rods themselves have a keyhole or paddle shape in cross-section in human teeth. The “head” of one rod nests between the “tails” of two adjacent rods. This interlocking pattern further enhances the enamel’s durability. Surrounding each rod is a thin layer called the rod sheath, which has a slightly higher concentration of organic material than the rod itself. The spaces between the rods, filled with more organic material, are known as interrod enamel.
The Making of a Shield: How Enamel Comes to Be
The formation of enamel, a process called amelogenesis, is a fascinating biological feat carried out by specialized cells called ameloblasts. This process occurs only during tooth development, before the tooth erupts into the mouth. Once the tooth has fully formed and erupted, the ameloblasts are lost, which is a critical point: enamel cannot regenerate or repair itself if it’s significantly damaged later in life.
Amelogenesis happens in stages:
- Presecretory Stage: Ameloblasts differentiate and prepare to produce enamel matrix proteins.
- Secretory Stage: Ameloblasts actively secrete enamel matrix proteins, primarily amelogenins and enamelins. This organic matrix provides a scaffold for mineralization. The hydroxyapatite crystals begin to form within this matrix, and the enamel layer grows in thickness.
- Maturation Stage: This is a crucial stage where the bulk of the organic matrix and water are removed, and more minerals are deposited. The crystals grow wider and thicker, leading to the extremely high mineralization and hardness of mature enamel. This stage involves cycles of modulation where ameloblasts alternate between ruffle-ended and smooth-ended forms, which are thought to be involved in removing protein and adding mineral.
The highly organized structure of enamel rods is a direct result of the precise and controlled activity of the ameloblasts during these stages.
A critical fact about tooth enamel is its inability to regenerate. Unlike skin or bone, which can heal and repair, enamel does not have living cells once tooth formation is complete. This means that any enamel lost due to decay, erosion, or wear is gone for good. Therefore, preserving the enamel you have is incredibly important for lifelong dental health.
Why Your Enamel is a Big Deal
The importance of enamel cannot be overstated. It serves several vital functions:
- Protection: As mentioned, its primary role is to shield the sensitive inner dentin and pulp from physical damage during biting and chewing, thermal shocks from hot or cold foods, and chemical attacks from acidic substances.
- Insulation: Enamel helps insulate the tooth from extreme temperatures, preventing the discomfort or pain that can occur when hot or cold substances directly contact the more sensitive dentin.
- Aesthetics: The smooth, translucent surface of healthy enamel contributes significantly to the appearance of your smile. Its ability to reflect and refract light gives teeth their characteristic luster.
- Facilitating Mastication: The hard, durable surface of enamel provides an effective grinding surface for breaking down food, which is the first step in digestion.
Without enamel, our teeth would be incredibly vulnerable, prone to sensitivity, rapid wear, and decay, making everyday functions like eating quite difficult and uncomfortable.
When the Shield Weakens: Threats to Enamel Integrity
Despite its incredible hardness, enamel is not invincible. It faces constant challenges in the oral environment. The main threats to enamel integrity are demineralization, erosion, and abrasion.
The Acid Attack: Demineralization and Caries
The most common threat is demineralization, which is the loss of minerals (primarily calcium and phosphate) from the enamel. This process is primarily driven by acids. These acids can come from two main sources:
- Bacterial Acids: Bacteria in dental plaque (a sticky film of bacteria that constantly forms on teeth) feed on sugars and starches from our diet. As they metabolize these carbohydrates, they produce acids as byproducts. These acids, if left in contact with the tooth surface, slowly dissolve the enamel minerals. This is the fundamental process behind dental caries, commonly known as cavities.
- Dietary Acids: Many foods and drinks are inherently acidic, such as citrus fruits, sodas, sports drinks, wine, and even some fruit juices. Frequent and prolonged exposure to these dietary acids can also directly erode enamel, even in the absence of significant bacterial action. This is known as dental erosion.
Initially, demineralization might appear as a chalky white spot on the enamel. If the process continues, the enamel structure weakens and can eventually break down, forming a cavity that may require professional dental treatment.
Wearing Away: Erosion and Abrasion
Dental erosion, as mentioned, is the chemical dissolution of enamel by acids not produced by bacteria. It’s often related to diet (frequent consumption of acidic foods and drinks) or medical conditions like acid reflux (GERD) or bulimia, where stomach acids regularly contact the teeth.
Abrasion is the physical wearing away of enamel due to mechanical forces other than chewing. This can be caused by:
- Overly aggressive tooth brushing, especially with a hard-bristled toothbrush or abrasive toothpaste.
- Using teeth as tools (e.g., to open packages or bite nails).
- Certain oral habits or improperly fitted dental appliances.
Attrition is another form of wear, specifically tooth-to-tooth contact, such as from grinding (bruxism) or clenching. While natural during chewing, excessive attrition can wear down enamel significantly over time.
Guarding Your Enamel Shield: Basic Protective Measures
Since enamel cannot regenerate, prevention is key to maintaining its health and integrity. While this article isn’t about providing medical advice, understanding the nature of enamel naturally leads to some generally accepted principles for its care:
- Managing Acid Exposure: Being mindful of the frequency and duration of exposure to acidic foods and drinks is important. Rinsing the mouth with water after consuming acidic items can help neutralize acids.
- Good Oral Hygiene: Regular and proper brushing with a soft-bristled toothbrush and fluoride toothpaste helps remove plaque and food debris, reducing the bacterial acid challenge. Flossing helps clean between teeth where brushes can’t easily reach.
- The Role of Fluoride: Fluoride, as discussed earlier, strengthens enamel by promoting remineralization (the natural repair process where minerals are redeposited into enamel) and making enamel more resistant to acid attacks. It’s found in many toothpastes, some mouth rinses, and can be applied professionally.
- Dietary Considerations: A diet balanced in nutrients and lower in sugary and starchy foods can reduce the fuel available for acid-producing bacteria.
- Regular Dental Check-ups: Professional cleanings remove hardened plaque (tartar) that brushing alone cannot, and check-ups can help identify early signs of enamel wear or demineralization when they are potentially easier to manage.
Your enamel is a marvel of biological engineering, designed to last a lifetime with proper care. Understanding its structure, how it’s formed, and what threatens it empowers you to take better care of this essential protective layer, ensuring your teeth stay strong and healthy for years to come.