We’ve all heard it, haven’t we? Reach for the sugar-free option, and your teeth will thank you. It’s a comforting thought, especially when that craving for something fizzy or flavored hits. The logic seems sound: no sugar means no fuel for those pesky cavity-causing bacteria. But what if this widely accepted belief isn’t the whole story? What if some of those ‘healthier’ choices are waging a silent war on your enamel? It’s time to look beyond the sugar content and uncover a different kind of threat lurking in many sugar-free drinks.
Let’s quickly recap why sugar gets such a bad rap in the dental world. When you consume sugary foods or drinks, bacteria that naturally live in your mouth have a feast. As they break down these sugars, they produce acids as a byproduct. These acids are the villains that attack your tooth enamel, the hard, protective outer layer of your teeth. Over time, repeated acid attacks can demineralize the enamel, creating weak spots that eventually turn into cavities. So, cutting out sugar logically seems like a direct route to fewer dental woes.
The Acidity Angle – More Than Just Sugar
Here’s where the sugar-free narrative gets a bit more complicated. While it’s true that removing sugar takes away the primary food source for acid-producing bacteria, many sugar-free drinks introduce acid directly into your mouth. The measure of acidity is called
pH. A pH of 7 is neutral (like pure water). Anything below 7 is acidic, and the lower the number, the more acidic the substance. Unfortunately, a significant number of sugar-free beverages register surprisingly low on the pH scale, meaning they are quite acidic.
Common culprits that contribute to this acidity include ingredients like citric acid (often found in citrus-flavored drinks), phosphoric acid (a common ingredient in colas, both regular and diet), and malic acid (found in some fruit-flavored drinks). These acids are added for tanginess, to enhance flavor, or even as preservatives. When these acidic drinks wash over your teeth, they can cause a problem called dental erosion. This is different from cavities (dental caries). Erosion is the direct chemical dissolution of tooth enamel by acids,
without any bacteria needing to be involved. It’s like an acid bath for your teeth, slowly wearing away that precious protective layer.
Many popular sugar-free beverages, including diet sodas and some flavored waters, contain high levels of acid. This acid can directly erode tooth enamel, leading to sensitivity and other dental problems. Always check ingredient lists for acids like citric, phosphoric, or malic acid, even if the label says ‘sugar-free’. Understanding the full ingredient list is key to making informed choices.
Not All Sugar-Free Sips Are Created Equal
It’s easy to lump all ‘sugar-free’ drinks together, but their impact on teeth can vary significantly, largely due to their acid content. Let’s look at some common categories:
- Diet Sodas: These are perhaps the most well-known sugar-free culprits. While they lack sugar, many diet colas contain phosphoric acid, and other diet sodas often use citric acid for flavor. Their pH levels can be surprisingly low, sometimes comparable to their sugary counterparts in terms of acidity.
- Sugar-Free Sports Drinks: Designed to rehydrate and replenish electrolytes, sports drinks often need to be palatable, and acidity contributes to their taste. Even the sugar-free versions can contain significant amounts of citric acid or other acidulants to achieve that tangy flavor profile.
- Sugar-Free Fruit Juices and Flavored Waters: Be wary here. ‘Fruit essence’ or ‘natural flavors’ can sometimes mean the inclusion of fruit acids. Some sugar-free versions of fruit drinks might have less sugar, but they can still pack an acidic punch from the fruit itself or added acids like citric acid to mimic the tartness of real fruit.
- Sparkling Water (Unsweetened, Unflavored): Plain carbonated water is generally a safer bet. The process of carbonation creates carbonic acid, which is a weak acid. While it does lower the pH slightly, making it more acidic than plain still water, it’s usually far less aggressive than the acids found in sodas or sports drinks. However, once flavorings (especially citrus) are added to sparkling water, the acidity can increase notably.
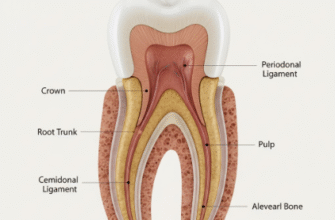
So, what exactly happens when these acids meet your enamel? Tooth enamel is the hardest substance in the human body, primarily made of a mineral called hydroxyapatite. Despite its strength, it’s vulnerable to acid. When the pH in your mouth drops below a critical level (around 5.5 for enamel), demineralization begins. This means the acid starts to dissolve the mineral crystals from the enamel surface. Unlike tooth decay, where bacteria create localized acid attacks leading to cavities, erosion is a more widespread surface dissolution. It’s a bit like sandpaper slowly wearing down a smooth surface. Over time, this gradual loss of enamel can lead to several noticeable issues: your teeth might become more sensitive to hot, cold, or sweet things because the protective enamel layer is thinner. They can appear more translucent, especially at the edges, or even take on a yellowish hue as the underlying, naturally yellower dentin layer becomes more visible. In more advanced cases, you might notice small indentations or ‘cupping’ on the chewing surfaces of your teeth.
It’s important to clarify the role of artificial sweeteners in this equation. Sweeteners like aspartame, sucralose, saccharin, or stevia do not, in themselves, cause tooth decay or erosion. Oral bacteria generally cannot metabolize these sweeteners to produce acid. So, from that specific perspective, they are indeed ‘tooth-friendly’ compared to sugar. The problem, as we’ve seen, isn’t the sweetener itself but the
overall acidic environment of the beverage it’s dissolved in. A drink can be completely free of sugar and sweetened with a non-nutritive sweetener, yet still be highly corrosive due to added acids for flavor, preservation, or fizz.
Smart Sipping Strategies to Protect Your Smile
Knowing about the acid risk in sugar-free drinks doesn’t mean you can never enjoy them, but it does mean being smarter about how and when you consume them. Here are some practical tips to help minimize potential enamel erosion:
- Mind the Frequency: It’s not just about what you drink, but how often. Every time you sip an acidic beverage, your mouth’s pH drops, and your enamel is under attack. Constant sipping throughout the day means your teeth are bathed in acid for extended periods, giving them little chance to recover. Try to limit these drinks to mealtimes if possible.
- Drink, Don’t Dwell: Avoid swishing acidic drinks around your mouth or holding them there for long periods. The less time the acid is in direct contact with your teeth, the better. Drink them relatively quickly.
- Use a Straw: While not a perfect solution, using a straw can help bypass some of your teeth, especially the front ones, directing the liquid more towards the back of your mouth. This can reduce direct acid contact.
- Rinse with Water Afterwards: After finishing an acidic drink, rinse your mouth thoroughly with plain water. This helps to wash away some of the residual acid and bring your mouth’s pH back towards neutral more quickly.
- Wait Before You Brush: This might sound counterintuitive, but brushing your teeth immediately after consuming something acidic can actually be more harmful. Acid softens the enamel temporarily, and the abrasive action of brushing can then scrub away this softened layer. It’s generally recommended to wait at least 30 to 60 minutes after an acidic drink before brushing to allow your saliva a chance to neutralize the acids and start the remineralization process.
- Choose Wisely When Possible: Plain water is always the best choice for hydration and tooth health. Unsweetened green or black tea (without lemon or other acidic additions) and milk are also generally safer options from an acidity standpoint.
- Read the Labels: Get into the habit of checking ingredient lists. Look for terms like ‘citric acid,’ ‘phosphoric acid,’ ‘malic acid,’ or even high concentrations of fruit juice concentrates, which can indicate a higher acidity level. This awareness is crucial.
Your body has a natural defense mechanism against acid attacks: saliva. Saliva plays a crucial role in protecting your teeth. It helps to wash away food particles and acids, neutralize the acidity in your mouth (acting as a buffer), and contains minerals like calcium and phosphate that can help to remineralize or repair early enamel damage. However, when acidic challenges are too frequent or too strong, saliva’s protective capabilities can be overwhelmed. This is why limiting the frequency and duration of acid exposure is so important – it gives your saliva a fighting chance to do its job.
The ‘sugar-free’ label can indeed be a positive step away from the cavity-causing potential of sugar. However, it’s a mistake to assume it automatically translates to ‘completely safe for teeth.’ The hidden factor of acidity in many popular sugar-free drinks poses a real threat of dental erosion, silently wearing away enamel even in the absence of sugar. By understanding this risk and adopting smarter consumption habits, you can make more informed choices that support not just your general well-being but also the long-term health and strength of your smile. After all, awareness is the first step towards better protection.