Imagine a shield, tougher than bone, diligently protecting the sensitive inner parts of your teeth day in and day out. This incredible natural armor is tooth enamel, the gleaming, hard outer layer that we see when we smile. It is the first line of defense against the daily onslaught of chewing, biting, and the diverse chemical environments introduced by the foods and drinks we consume. Without this remarkable substance, our teeth would be far more vulnerable to damage and decay, making everyday eating a painful, if not impossible, task.
The Building Blocks of a Super Shield
The secret to enamel is incredible strength and resilience lies in its unique composition. It is a highly mineralized structure, in fact, the most mineralized tissue produced by the body. Think of it as a natural ceramic, meticulously crafted for durability.
A Mineral Masterpiece: Hydroxyapatite
The vast majority of enamel, around 96 percent by weight, is made up of an inorganic mineral called
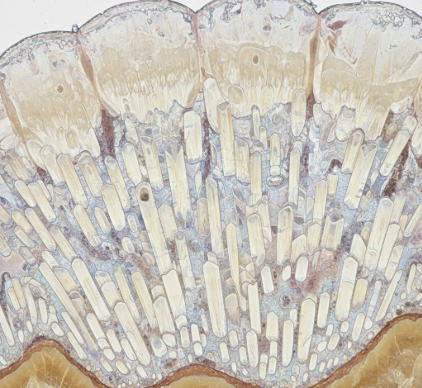
hydroxyapatite. This is a crystalline calcium phosphate. Its complex crystal lattice is formed from calcium ions, phosphate ions which themselves are combinations of phosphorus and oxygen atoms, and hydroxide ions which are combinations of oxygen and hydrogen atoms. These components are organized into tightly packed, elongated structures known as enamel rods or prisms. This specific arrangement is crucial, contributing significantly to enamel is ability to withstand immense forces. Imagine microscopic bundles of incredibly strong rods, all aligned to provide maximum resistance. Other minerals, such as fluoride, carbonate, and magnesium, are also present in trace amounts, subtly influencing enamel is properties, like its solubility in acids.
The Supporting Cast: Organics and Water
While minerals take center stage, a small percentage of enamel, roughly one to two percent, consists of organic materials. These are primarily proteins, with names like amelogenins, enamelins, and tuftelin. These proteins play a vital role during the formation of enamel, a process known as
amelogenesis. They act as a scaffold or framework, guiding the deposition and organization of the hydroxyapatite crystals. Once enamel is fully mature, much of this organic matrix is removed, leaving behind a predominantly mineral structure.
The remaining few percent, around two to three percent, is water. This water is found within the tiny spaces between the mineral crystals and associated with the organic components. While a small fraction, it still plays a part in the enamel is overall dynamics, including its slight permeability.
Its composition endows tooth enamel with a set of properties that make it perfectly suited for its demanding job. It is not just about being hard; it is a sophisticated interplay of characteristics.
The Hardness Factor
Tooth enamel proudly holds the title of the
hardest substance in the human body. On the Mohs scale of mineral hardness, it scores a five, making it comparable to apatite mineral, and significantly harder than dentin, the layer beneath enamel, or bone. This exceptional hardness is a direct result of its high mineral content and the dense, crystalline structure of hydroxyapatite. It is what allows our teeth to grind, crush, and chew a wide variety of foods without rapidly wearing away.
A Delicate Balance: Brittleness and Support
Interestingly, despite its incredible hardness, enamel is also quite
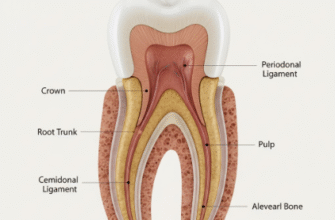
brittle. If it were a standalone material, it might chip or fracture easily under sharp impacts. However, nature has a clever design. Enamel is firmly bonded to the underlying dentin. Dentin is softer and more elastic than enamel, acting like a cushion or shock absorber. This supportive layer of dentin helps to distribute forces and prevent enamel from cracking under pressure. It is a fantastic example of two different materials working synergistically.
The Science of a Smile: Translucency
Enamel itself is not perfectly opaque; it is actually
translucent, meaning light can pass through it to some extent. The color of our teeth is therefore not solely determined by the enamel, but significantly influenced by the color of the dentin underneath, which is typically a more yellowish hue. The thickness and mineral density of the enamel can affect how much of the dentin color shows through. Thinner or less mineralized enamel might make teeth appear more yellow or grey, as the dentin is color becomes more prominent.
A Non Living Shield: Acellular Nature
One of the most critical properties of mature enamel is its
acellular nature. During its formation, specialized cells called ameloblasts are responsible for secreting the proteins and minerals that make up enamel. However, once the tooth erupts into the mouth and enamel formation is complete, these ameloblast cells are lost. This means that, unlike bone or dentin which contain living cells, mature enamel has no cellular components. This has a profound implication:
Because enamel contains no living cells, it lacks the ability to regenerate or repair itself in the way that other tissues, like skin or bone, can. Significant damage from decay or trauma is permanent. This underscores the importance of preserving the enamel we have through careful attention and good habits. Therefore, any substantial loss of enamel cannot be naturally restored by the body.
Slightly Spongy: Permeability
While incredibly dense, enamel is not entirely impermeable. It possesses a degree of
microporosity, allowing very small ions and molecules, including water, to pass through it. This permeability is a double edged sword. On one hand, it allows acids produced by bacteria to penetrate the enamel and dissolve minerals, leading to demineralization, the first step of tooth decay. On the other hand, it also allows beneficial ions, like fluoride, calcium, and phosphate from saliva or oral care products, to enter the enamel and promote remineralization, helping to repair early lesions.
Resisting the Acid Test Up to a Point
Thanks to its dense mineral structure, enamel exhibits considerable
resistance to chemical attack, particularly from acids. However, this resistance is not absolute. When the pH in the mouth drops below a certain critical level, around 5.5 for hydroxyapatite, enamel begins to demineralize. This acidic environment can be created by bacteria metabolizing sugars or by consuming acidic foods and drinks directly. The presence of fluoride can make enamel more acid resistant by converting some hydroxyapatite into fluorapatite, which dissolves at a lower pH.
Crafting the Armor: A Glimpse into Amelogenesis
The creation of tooth enamel, or
amelogenesis, is a fascinating and complex biological process orchestrated by those specialized cells mentioned earlier: the ameloblasts. These cells go through various stages, meticulously secreting specific proteins that form an organic matrix. This matrix then acts as a template for the precise deposition and crystallization of hydroxyapatite minerals. The ameloblasts control the size, shape, and orientation of these crystals, leading to the highly organized rod like structure of mature enamel. As the enamel matures, much of the initial protein and water is removed, and the mineral crystals grow and pack more tightly, resulting in the incredibly hard final product. Once their job is done and the tooth is ready to emerge, the ameloblasts undergo a programmed cell death or become part of the reduced enamel epithelium, eventually being lost. This final step seals enamel is fate as a non vital, non regenerative tissue.
Forces Working Against Your Enamel
Despite its formidable nature, enamel faces constant challenges throughout our lives. Understanding these can help appreciate its limits. One major adversary is
acid. This can come from two main sources: intrinsic, like stomach acids in individuals with acid reflux, or extrinsic, from the frequent consumption of acidic foods and beverages like citrus fruits, sodas, and sports drinks. This chemical erosion slowly dissolves the mineral content of enamel, making it thinner and weaker over time.
Another significant factor is the mechanical force of
attrition (tooth to tooth wear, often exacerbated by grinding or clenching, known as bruxism) and
abrasion (wear from foreign objects, like overly aggressive toothbrushing or abrasive toothpastes). While designed to withstand chewing, excessive or unnatural forces can gradually wear down the enamel surface. The interplay between chemical erosion and mechanical wear can be particularly damaging, as softened enamel is more susceptible to being worn away.
Of course, the most commonly discussed threat is dental caries, or tooth decay. This process is initiated by specific bacteria in dental plaque that metabolize dietary sugars and starches, producing acids as byproducts. These acids, concentrated within the plaque biofilm, repeatedly attack the enamel surface, leading to demineralization. If this process outpaces the mouth is natural remineralization capabilities, a cavity will form.
Guarding Your Natural Shield
Given enamel is inability to regenerate, its preservation is paramount. While we cannot regrow lost enamel, we can certainly take steps to protect what we have and support the natural defense mechanisms of our mouths. Managing dietary acid exposure is a key strategy. This involves being mindful of the frequency and duration of contact with acidic foods and drinks. Rinsing the mouth with water after consuming acidic items can help neutralize acids and wash them away.
The science behind fluoride is also compelling in enamel protection. Fluoride ions can integrate into the hydroxyapatite crystal lattice, forming
fluorapatite. Fluorapatite is inherently more resistant to acid dissolution than hydroxyapatite, meaning it starts to demineralize at a lower, more acidic, pH. Fluoride also enhances the remineralization process, helping to rebuild mineral content in areas that have been slightly demineralized. Saliva itself plays a crucial role, acting as a buffer to neutralize acids, providing calcium and phosphate for remineralization, and washing away food debris.
Gentle but thorough oral hygiene practices help remove the bacterial plaque that produces harmful acids. This minimizes the direct acid attack on the enamel surface. The goal is to disrupt the biofilm and reduce the bacterial load responsible for initiating the demineralization process.
The Enduring Marvel of Tooth Enamel
Tooth enamel stands as a true marvel of biological engineering. Its intricate composition, dominated by perfectly arranged hydroxyapatite crystals, gives rise to an extraordinary combination of hardness, resilience, and functional elegance. From its role in withstanding the immense forces of mastication to providing the aesthetic gleam of a healthy smile, enamel performs its duties tirelessly. While it faces daily assaults from chemical and mechanical forces, understanding its structure and properties empowers us to better appreciate and protect this irreplaceable, non living shield. It is a silent guardian, a testament to nature is ingenuity, and a critical component of our overall well being.