The journey of a tooth, from a mere speck of cellular potential to a fully formed structure, is a marvel of biological engineering. It’s a process orchestrated with incredible precision, guided by a complex symphony of genetic instructions. When we smile, chew, or speak, we rarely consider the intricate developmental dance that gave rise to our teeth. Yet, understanding this process, known as odontogenesis, is key not only to appreciating dental health but also to unraveling why things sometimes go astray, leading to various dental anomalies.
The Blueprint of a Tooth: Stages of Development
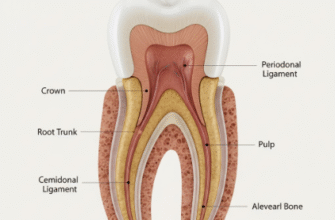
Tooth development doesn’t happen overnight. It’s a carefully staged process, each phase building upon the last, heavily reliant on a dialogue between two primary cell layers: the epithelium (which will give rise to enamel) and the underlying mesenchyme (which forms dentin, pulp, and cementum).
Initiation: The Spark of Life
It all begins with the initiation stage. Specific regions of the oral epithelium thicken, forming a band called the primary epithelial band. This band then gives rise to the dental lamina, a sheet of cells that dips into the mesenchyme, marking the future locations of our teeth. Think of it as laying down the initial foundation stones for a very complex building.
Bud, Cap, and Bell: Shaping Up
Following initiation, the dental lamina proliferates at specific points, forming little protrusions known as tooth buds. This is the bud stage. As development progresses, the epithelial bud continues to grow and invaginate, taking on a concave appearance, resembling a cap – hence the cap stage. During this time, the mesenchymal cells condense around this epithelial cap, forming the dental papilla (future dentin and pulp) and the dental follicle (future cementum and periodontal ligament).
The subsequent bell stage is where things get even more sophisticated. The epithelial component, now called the enamel organ, differentiates into distinct cell layers: the inner enamel epithelium (ameloblasts, which will form enamel), the outer enamel epithelium, the stellate reticulum, and the stratum intermedium. The overall shape of the tooth crown is determined during this critical morphodifferentiation phase. Simultaneously, histodifferentiation occurs, where cells specialize into their functional roles.
Hard Work: Apposition and Calcification
Once the crown shape is established, the hard tissues begin to form in the apposition stage. Ameloblasts start depositing enamel matrix, while odontoblasts (differentiated from the dental papilla) lay down dentin. This happens layer by layer. Mineralization, or calcification, follows shortly after matrix deposition, hardening these tissues. It’s a meticulously timed process, ensuring the correct structure and strength.
Making an Entrance: Eruption and Root Formation
Root formation only begins after crown formation is largely complete. The Hertwig’s epithelial root sheath (HERS), an extension of the enamel organ, guides root development. As the root lengthens, the tooth begins its journey towards the oral cavity in a process called eruption. This isn’t just a simple push; it involves complex interactions with the surrounding bone and tissues.
The Genetic Conductors of Odontogenesis
The entire saga of tooth development is under tight genetic control. Hundreds, yes, hundreds of genes are involved, acting like a vast orchestra where each musician must play their part at the precise moment. These genes encode for various proteins, including growth factors, transcription factors, signaling molecules, and structural proteins, all coordinating the cellular activities of proliferation, differentiation, adhesion, migration, and apoptosis (programmed cell death).
Signaling Pathways: The Communication Network
Critical to this coordination are several highly conserved signaling pathways. Imagine these as communication networks, allowing cells to talk to each other and respond appropriately. Key players include:
- Fibroblast Growth Factor (FGF) pathway: Involved in early initiation and patterning.
- Bone Morphogenetic Protein (BMP) pathway: Crucial for cell differentiation and morphogenesis.
- Sonic Hedgehog (SHH) pathway: Plays a role in tooth bud formation and cusp patterning.
- WNT pathway: Essential for tooth initiation and the continuous development of replacement teeth in some species.
These pathways don’t work in isolation; they interact extensively, creating intricate feedback loops that fine-tune development.
Transcription Factors: The Master Regulators
Transcription factors are proteins that bind to specific DNA sequences, controlling the rate at which genetic information is transcribed into messenger RNA, and thus, how much of a protein is made. They are the master regulators, switching genes on or off at the right time and place. Some of the most well-studied transcription factors in tooth development include:
- MSX1 and MSX2: Vital for the early stages, particularly in the mesenchyme. Mutations can lead to missing teeth.
- PAX9: Another key player in tooth agenesis (missing teeth), working in concert with MSX1.
- RUNX2: Essential for bone and tooth development, including the formation of supernumerary teeth when its function is altered, as seen in cleidocranial dysplasia.
- DLX1 and DLX2: Involved in patterning and morphogenesis.
- PITX2: Crucial for tooth initiation and overall craniofacial development.
The precise expression patterns of these factors – where and when they are active – dictate the identity, number, and shape of teeth.
Epithelial-Mesenchymal Crosstalk: A Dynamic Duo
A hallmark of tooth development is the continuous, reciprocal signaling between the epithelium and the mesenchyme. This epithelial-mesenchymal interaction is a two-way street. The epithelium sends signals to the mesenchyme, influencing its behavior, and the mesenchyme, in turn, signals back to the epithelium. This dialogue is mediated by the signaling pathways and transcription factors mentioned above and is absolutely fundamental for every stage, from initiation to the formation of complex crown patterns.
The development of our teeth is a remarkably intricate biological process, governed by the precise expression and interaction of hundreds of genes. This genetic complexity underscores why variations or mutations can lead to such a diverse array of dental anomalies. Understanding this genetic framework is fundamental for diagnosing, and perhaps one day predicting, these conditions with greater accuracy.
When the Genetic Blueprint Has Errors: Dental Anomalies
Given the sheer complexity of tooth development and the vast number of genes involved, it’s perhaps not surprising that things can sometimes go off track. Genetic mutations or variations can disrupt this delicate process, leading to a wide spectrum of dental anomalies affecting the number, size, shape, or structure of teeth.
Too Few or Too Many: Anomalies of Number
One of the most common groups of dental anomalies relates to tooth number. Hypodontia refers to the congenital absence of one or more teeth. When six or more teeth (excluding third molars) are missing, it’s termed oligodontia, and the complete absence of teeth is anodontia (which is very rare). Genes like MSX1, PAX9, AXIN2, EDA, and WNT10A are frequently implicated. Mutations in these genes can disrupt signaling pathways crucial for tooth initiation.
Conversely, supernumerary teeth (or hyperdontia) involve the development of extra teeth. These can occur anywhere in the dental arches, with the mesiodens (an extra tooth between the maxillary central incisors) being a common example. While the exact genetic causes for isolated supernumerary teeth are often complex and less clear-cut, they are a common feature in certain genetic syndromes, such as cleidocranial dysplasia, linked to RUNX2 mutations, and Gardner syndrome, associated with APC gene mutations.
Size Matters: Microdontia and Macrodontia
Microdontia describes teeth that are smaller than normal, which can be localized (e.g., “peg laterals,” often affecting maxillary lateral incisors) or generalized. Macrodontia refers to teeth that are larger than normal. While hormonal influences and environmental factors can play a role, genetic factors are often involved, particularly in generalized forms or when associated with syndromes. The genes controlling overall growth and signaling pathways that regulate cell proliferation during the cap and bell stages are likely candidates.
Odd Shapes and Forms: Anomalies of Morphology
Teeth can also develop with unusual shapes:
- Taurodontism: Characterized by an enlarged pulp chamber and apically displaced furcation of the roots, giving the tooth a bull-like appearance. It can be an isolated trait or part of a syndrome.
- Fusion and Gemination: Fusion is the union of two normally separate tooth germs, resulting in a single large tooth, often with two root canals. Gemination occurs when a single tooth germ attempts to divide, resulting in a large tooth with a single root and canal, but a partially or completely bifid crown. Differentiating these can be tricky.
- Dens Evaginatus and Dens Invaginatus (Dens in Dente): Dens evaginatus is an extra cusp or tubercle, while dens invaginatus is an infolding of the enamel and dentin, sometimes extending deep into the tooth.
The genetic basis for many isolated shape anomalies is still being investigated, but they often arise from disruptions during the morphodifferentiation phase of the bell stage.
Structural Integrity Issues: Problems with Enamel and Dentin
Defects in the formation of the hard tissues of the tooth are also genetically rooted.
Amelogenesis Imperfecta (AI) is a group of hereditary conditions characterized by defects in enamel formation. The enamel can be thin, soft, pitted, or discolored. It can affect all teeth in both dentitions. Mutations in several genes are known to cause AI, including AMELX (encoding amelogenin, the major enamel matrix protein), ENAM (enamelin), MMP20 (matrix metalloproteinase-20, involved in degrading enamel proteins), and KLK4 (kallikrein-4, also involved in protein processing during enamel maturation). These genes are crucial for different stages of amelogenesis (enamel formation).
Dentinogenesis Imperfecta (DI) affects dentin formation, leading to discolored (often opalescent blue-gray or brownish) teeth that are prone to wear and fracture. The pulp chambers may be obliterated. Most forms of DI are caused by mutations in the DSPP (dentin sialophosphoprotein) gene. This gene codes for a precursor protein that is cleaved into dentin sialoprotein (DSP) and dentin phosphoprotein (DPP), both critical for dentin mineralization.
Dentin Dysplasia (DD) is another group of inherited dentin disorders. Type I (radicular) is characterized by short or absent roots and obliterated pulp chambers, while Type II (coronal) resembles DI in the primary dentition, but the permanent teeth have normal color with thistle-tube shaped pulp chambers. DD is also linked to mutations in the DSPP gene.
Part of a Bigger Picture: Syndromic vs. Non-Syndromic Anomalies
Dental anomalies can occur as isolated traits, referred to as non-syndromic. For instance, a person might have a single missing tooth or a case of amelogenesis imperfecta without any other associated health issues. In these cases, the genetic mutation primarily affects tooth development.
However, dental anomalies are also frequently features of broader genetic syndromes, where multiple organ systems are affected. There are hundreds of such syndromes. For example:
- Ectodermal Dysplasias: A large group of disorders affecting tissues derived from the ectoderm, including skin, hair, nails, sweat glands, and teeth. Hypodontia or anodontia are common features. Mutations in genes like EDA, EDAR, and WNT10A are often involved.
- Cleidocranial Dysplasia: Caused by mutations in RUNX2, this syndrome affects bone and tooth development, leading to features like unerupted permanent teeth, supernumerary teeth, and absent or poorly developed clavicles.
- Down Syndrome (Trisomy 21): Individuals with Down syndrome often exhibit various dental anomalies, including hypodontia, microdontia, delayed eruption, and malocclusion. The exact genetic mechanisms for these specific dental features within the context of an extra chromosome 21 are still being explored.
- Osteogenesis Imperfecta: Primarily a brittle bone disease, some types are associated with dentinogenesis imperfecta due to mutations in collagen genes (COL1A1, COL1A2), as collagen is also a key component of dentin.
Recognizing dental anomalies can sometimes be an important clue in diagnosing an underlying syndrome, highlighting the interconnectedness of developmental processes throughout the body.
Unlocking Secrets: Research and Future Perspectives
The field of dental genetics is dynamic and continually evolving. Researchers are employing sophisticated techniques to identify new genes involved in tooth development and anomalies. Genome-Wide Association Studies (GWAS) scan the entire genome of many individuals to find genetic variations associated with a particular trait or disease, like hypodontia. Next-Generation Sequencing (NGS) technologies, including whole-exome and whole-genome sequencing, allow for rapid and comprehensive analysis of an individual’s genetic makeup, proving invaluable in identifying causative mutations, especially for rare conditions.
Animal models, particularly mice, play a crucial role. Many of the genes involved in tooth development are conserved across species, so studying mice with specific gene mutations allows researchers to understand the gene’s function in a living system and how its disruption leads to dental defects. This provides insights that are often not possible through human studies alone.
While direct gene therapies for common dental anomalies are still a distant prospect and fraught with ethical and practical challenges, ongoing research holds promise for improved diagnostic capabilities. Understanding the specific genetic cause of an anomaly can lead to more accurate risk assessment for families and can inform personalized approaches to dental care. For instance, knowing the genetic basis of a severe form of amelogenesis imperfecta might guide decisions about preventative measures and the timing and type of restorative treatments.
Furthermore, a deeper understanding of the fundamental genetic and molecular mechanisms of normal tooth development could one day contribute to regenerative dentistry approaches, aiming to bioengineer tooth structures or even whole teeth. This is a long-term vision, but it’s one fueled by the intricate knowledge being gained from genetic studies.
The Intricate Genetic Ballet
The formation of our teeth is a testament to the power and precision of genetic programming. From the first epithelial thickening to the eruption of a fully mineralized crown, an elaborate molecular ballet unfolds, directed by hundreds of genes working in concert. When this genetic choreography is disturbed, the result can be a wide spectrum of dental anomalies, impacting not just oral function and aesthetics, but sometimes signaling broader systemic conditions.
Exploring the genetic basis of tooth formation and its deviations continues to be a fascinating area of research. Each new gene identified, each pathway elucidated, brings us closer to understanding this fundamental aspect of our biology, paving the way for better insights and potentially new strategies to manage dental health in the future. Our smiles, in essence, are a direct reflection of a deeply complex genetic legacy.