The outer layer of our teeth, known as enamel, stands as a testament to nature’s incredible engineering. It’s the hardest substance in the human body, a crystalline shield protecting the sensitive inner parts of the tooth from the daily onslaught of chewing, temperature changes, and varying chemical environments. But what gives enamel this remarkable resilience? The answer lies in its intricate microstructure, built primarily from tiny, densely packed units called enamel rods or prisms. Delving into the world of these rods reveals a fascinating story of biological precision and structural ingenuity.
Unpacking the Enamel Rod
Defining the Microscopic Fiber
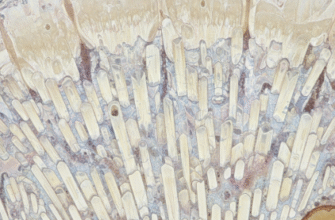
At its core, an enamel rod, sometimes referred to as an enamel prism, is an elongated, tightly bundled collection of hydroxyapatite crystals. Imagine them as microscopic crystalline fibers, with literally millions of them meticulously arranged and packed together to constitute the vast majority of the enamel layer. The sheer number and density are staggering when one considers the scale.
Dimensions and Extent
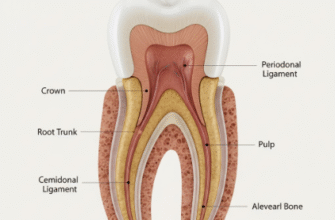
Each individual rod is a remarkable structure in itself, typically stretching uninterrupted from the dentino-enamel junction (DEJ) – the critical boundary where enamel meets the softer, underlying dentin – all the way out to the tooth’s external surface. Their dimensions are truly minuscule, generally measuring around 4 to 8 micrometers in diameter. Despite this tiny size, their collective organization and inherent strength contribute significantly to the overall robustness of enamel. The specific shape and intricate orientation of these rods are far from random; they are fundamental to enamel’s unique mechanical and optical properties.
The Genesis of Strength: Amelogenesis
A Masterclass in Biomineralization
The formation of enamel, a process called amelogenesis, is a remarkable feat performed by specialized cells known as ameloblasts. These cells are present only during tooth development, and once their job is done and the tooth erupts, they are lost forever. This means enamel, unlike bone, cannot regenerate or repair itself if significantly damaged. During amelogenesis, ameloblasts secrete proteins, primarily amelogenin, which create an organic matrix. This matrix acts as a scaffold, guiding the organized deposition and growth of hydroxyapatite crystals. Each ameloblast is responsible for forming a single enamel rod. The unique, retreating movement of the ameloblasts as they lay down the crystal-rich matrix dictates the rod’s elongated shape and its orientation within the enamel layer. It’s a slow, painstaking process, ensuring each rod is perfectly formed and integrated with its neighbors.
Inside an Enamel Rod: Crystalline Architecture
The Hydroxyapatite Backbone
The primary constituent of enamel rods – indeed, about 96% of enamel by weight – is a highly mineralized form of calcium phosphate called hydroxyapatite (Ca10(PO4)6(OH)2). Within each rod, these hydroxyapatite crystals are not randomly scattered. Instead, they are extremely long, thin, and needle-like or plate-like, and they are packed together with remarkable parallelism. The long axes of these crystals are generally aligned with the long axis of the rod itself, particularly in the core or head of the rod. This specific orientation is crucial. When forces are applied to the tooth, the alignment of these hard crystals within the rods helps to distribute stress effectively. The small amount of remaining material consists of water and organic matrix (proteins like enamelin and tuftelin), which, while minor in quantity, play roles in crystal nucleation and organization, and are found predominantly in the spaces between crystals and between rods (the interrod substance or sheath region).
Enamel rods are primarily composed of highly organized hydroxyapatite crystals. The long axes of these crystals are generally parallel to the long axis of the rod. This precise crystalline arrangement is fundamental to enamel’s exceptional hardness and its ability to withstand significant masticatory forces.
Interwoven Strength: Patterns in Enamel
The true genius of enamel’s design becomes apparent when we examine how these individual rods are arranged collectively. They aren’t just stacked like simple logs. Instead, they often exhibit complex, interwoven patterns that significantly enhance the tissue’s resistance to fracture.
The Keyhole Configuration
In many areas of human enamel, particularly in cross-section, enamel rods display a characteristic “keyhole” or “paddle” shape. Each rod has a wider “head” region and a narrower “tail” region. The heads of the rods are typically oriented towards the occlusal or incisal surface of the tooth, while the tails point cervically. The head of one rod nestles between the tails of the two adjacent rods in the row below. This interlocking arrangement minimizes empty space and creates a densely packed structure, contributing to enamel’s overall hardness.
Hunter-Schreger Bands: Resisting Cracks
When viewed under reflected light or certain microscopic techniques, enamel often reveals alternating light and dark bands known as Hunter-Schreger Bands (HSB). These bands are an optical phenomenon resulting from changes in the orientation of groups of enamel rods. Bundles of rods run in slightly different directions – some cut in cross-section (diazones) and others longitudinally (parazones) relative to the plane of view. This decussation, or weaving pattern of rod groups, acts as a crack-stopping mechanism. If a crack starts to propagate through the enamel, it encounters these alternating orientations, which deflect the crack’s path and dissipate its energy, making it much harder for the fracture to spread catastrophically through the entire enamel layer. It’s like the grain in wood, but far more sophisticated.
Gnarled Enamel: Ultimate Protection
At the cusps of teeth, the areas subjected to the most intense chewing forces, the enamel rods exhibit an even more complex and irregular arrangement known as “gnarled enamel.” Here, the rods become highly twisted and intertwined. This seemingly chaotic structure provides maximum resistance to fracture in these critical high-stress zones. The irregular paths of the rods make it incredibly difficult for cracks to find a straight path, thereby enhancing the durability of the biting surfaces.
Derived Characteristics: A Consequence of Design
The sophisticated architecture woven from countless enamel rods directly translates into the renowned physical characteristics of enamel. This isn’t just a random collection of properties; they are the logical outcomes of its meticulously ordered microstructure, especially its high mineral content and the specific arrangement of the hydroxyapatite crystals within and between the rods.
The Hardness Factor
Its most celebrated feature, exceptional hardness, is primarily due to this high mineral content – around 96% hydroxyapatite – and the dense packing of these crystals within the rods. This incredible hardness allows enamel to resist wear and tear from the abrasive forces encountered during mastication, effectively shielding the more vulnerable dentin and pulp beneath. It’s a frontline defense, built to endure.
The Trade-off: Brittleness
However, this impressive hardness comes with a trade-off: enamel is also quite brittle. Materials that are extremely hard are often less able to deform under stress and can be prone to fracture. While enamel is very strong under compressive forces (like direct biting), it can chip or crack if subjected to sharp, focused impacts or significant bending forces. This brittleness is somewhat mitigated by the softer, more resilient dentin foundation, which provides a degree of cushioning. The intricate patterns like Hunter-Schreger bands also play a vital role in arresting cracks, but the inherent nature of such a highly mineralized material includes this susceptibility.
Optical Qualities: Translucency
Beyond its mechanical strengths, the structure of enamel rods also influences its optical properties, notably its translucency. The crystalline nature of hydroxyapatite and the orderly arrangement of rods allow light to pass through the enamel to varying degrees. This translucency contributes to the aesthetic appearance of teeth, with the color of the underlying dentin playing a significant role in the overall perceived tooth shade. The way light interacts with the rod structure, including reflections and refractions at rod boundaries, adds to the vitality of a natural tooth’s appearance.
The enamel rod, therefore, is far more than just a simple component. It is a marvel of micro-engineering, a fundamental unit that, through its composition, shape, and intricate arrangement with its neighbors, bestows upon enamel its extraordinary ability to serve as a durable, protective armor for our teeth. Understanding these tiny building blocks provides a deeper appreciation for the complexity and elegance of biological structures, showcasing how nature optimizes materials for demanding functional roles. The study of enamel rods continues to inspire material scientists looking to create new, resilient materials, learning from a design perfected over millennia.