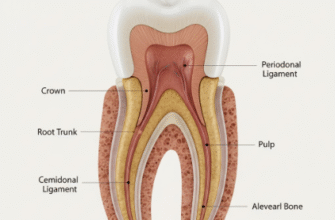
Our teeth are truly remarkable structures, and their outermost layer, the dental enamel, stands as a testament to nature’s engineering. This incredible material is the hardest substance in the human body, even tougher than bone. Its primary role is to protect the sensitive inner parts of the tooth from the daily onslaught of chewing forces, temperature changes, and, crucially, chemical attacks. Think of it as the tooth’s personal armor, a shimmering, resilient shield.
Composed almost entirely of minerals, predominantly in the form of hydroxyapatite crystals, enamel is a densely packed, highly organized structure. These crystals are long and rod-like, bundled together in intricate patterns, giving enamel its unique strength and translucency. It is this very composition, however, that also presents a specific vulnerability when confronted with certain chemical environments.
The Unseen Challenge: Understanding Acid Erosion
Despite its formidable hardness, enamel is not invincible. Its Achilles heel is acid. An “acid attack” is not a dramatic, sudden event but rather a subtle, ongoing chemical process that can gradually wear down this protective layer. This process is known as demineralization. When enamel is exposed to acidic conditions, the pH level at the tooth surface drops. If it falls below a critical point (typically around pH 5.5 for hydroxyapatite), the mineral crystals begin to dissolve, leaching calcium and phosphate ions from the enamel into the surrounding environment.
Where do these hostile acids come from? They primarily originate from two sources:
- Dietary Acids: Many foods and beverages we enjoy are inherently acidic. Citrus fruits, sodas, sports drinks, wine, and even some fruit juices can introduce a significant acid challenge to our teeth. The frequency and duration of exposure to these dietary acids play a significant role in the extent of potential demineralization.
- Bacterial Acids: Our mouths are home to a complex ecosystem of bacteria, some of which are harmless or even beneficial. However, certain types of bacteria, particularly Streptococcus mutans, thrive on sugars and fermentable carbohydrates from our diet. As they metabolize these sugars, they produce acidic byproducts, primarily lactic acid. This acid, concentrated within dental plaque (a sticky film of bacteria), directly bathes the tooth surface, leading to localized demineralization.
This gradual loss of mineral content weakens the enamel structure, making it softer and more prone to wear and further attack. It is a microscopic battle constantly being waged on the surfaces of our teeth.
Nature’s Defense System: How Enamel Fights Back
Fortunately, our bodies have evolved sophisticated mechanisms to counteract these acidic onslaughts and protect dental enamel. The primary line of defense is not just the enamel itself, but its dynamic interaction with saliva and the beneficial effects of certain minerals like fluoride.
The Power of Saliva: An Unsung Hero
Saliva is far more than just water; it is a complex biological fluid with several crucial protective functions for enamel:
- Buffering Capacity: Saliva contains bicarbonate and phosphate ions that act as buffers, neutralizing acids present in the mouth from food or bacterial metabolism. This helps to quickly raise the pH back to safer levels, halting the demineralization process.
- Clearing Action: The physical flow of saliva helps to wash away food particles and acids from tooth surfaces, reducing the duration of their contact with enamel. Think of it as a natural rinsing mechanism.
- Mineral Reservoir: Saliva is supersaturated with calcium and phosphate ions – the very building blocks of enamel. When conditions are favorable (i.e., when the pH is not too low), these minerals can be re-deposited onto partially demineralized enamel, a process called remineralization. This can repair microscopic lesions before they become significant.
- Pellicle Formation: Within minutes of teeth being cleaned, a thin, organic film called the acquired pellicle forms on the enamel surface. This layer, derived from salivary glycoproteins, acts as a selectively permeable barrier, offering some protection against direct acid attack and also providing a surface for beneficial bacteria to attach, potentially outcompeting more harmful strains.
Fluoride: The Mineral Enhancer
Fluoride is a naturally occurring mineral that has a profound impact on enamel’s resistance to acid. When fluoride is present in the oral environment, even in low concentrations, it can significantly enhance enamel’s defenses:
- Formation of Fluorapatite: During remineralization, if fluoride ions are available, they can become incorporated into the enamel crystal lattice, forming fluorapatite instead of, or alongside, hydroxyapatite. Fluorapatite is inherently more resistant to acid dissolution than hydroxyapatite; its critical pH for demineralization is lower (around pH 4.5). This means it takes a stronger acid attack to start dissolving fluorapatite-rich enamel.
- Enhanced Remineralization: Fluoride acts as a catalyst for remineralization. It attracts calcium and phosphate ions from saliva to the tooth surface, speeding up the repair of early demineralization sites.
- Inhibition of Bacterial Metabolism: At higher concentrations, fluoride can also interfere with the metabolic processes of acid-producing bacteria in plaque, reducing the amount of acid they produce.
Saliva plays a multifaceted role in protecting teeth. It not only neutralizes acids and washes away debris but also delivers essential minerals like calcium and phosphate. This constant supply of minerals is vital for the remineralization process, which helps repair early enamel damage caused by acid attacks. The dynamic balance between demineralization and remineralization is key to enamel health.
When Defenses Falter: Factors Undermining Enamel Strength
While enamel possesses remarkable resilience, certain conditions and habits can overwhelm its natural defenses, tipping the balance towards demineralization and erosion. Understanding these factors is key to appreciating the delicate equilibrium that maintains enamel integrity.
Frequent Acid Exposure: The most direct threat is, unsurprisingly, repeated and prolonged contact with acids. Sipping on sugary or acidic drinks throughout the day, or frequent snacking on acidic foods, means the enamel is constantly under attack. Saliva may not have enough time to buffer the acids and remineralize the tooth surface between exposures, leading to a net loss of mineral.
Low Salivary Flow (Xerostomia): A reduction in saliva production, a condition known as xerostomia or dry mouth, significantly compromises all the protective benefits saliva offers. Without adequate saliva, acids are not efficiently neutralized or cleared, and the supply of minerals for remineralization is diminished. This can be caused by certain medications, medical conditions, or dehydration.
High Plaque Levels: Poor oral hygiene allows dental plaque to accumulate. This thick biofilm harbors acid-producing bacteria right against the tooth surface. The acids produced within the plaque are concentrated and less easily neutralized by saliva, leading to sustained demineralization in those areas.
Abrasive Forces: While enamel is hard, it can be physically worn away, especially if it is already slightly softened by acid. Aggressive tooth brushing with a hard-bristled brush, or using highly abrasive toothpastes, can contribute to enamel loss over time. Similarly, conditions like bruxism (teeth grinding) can exert excessive forces that wear down enamel, particularly on the chewing surfaces.
Dietary Choices Beyond Acidity: While direct acidity is a major concern, a diet high in fermentable carbohydrates (sugars and starches) provides ample fuel for acid-producing bacteria. The more sugar available, the more acid these bacteria can generate, creating a persistently hostile environment for enamel.
Whispers of Wear: Recognizing Early Enamel Changes
When the balance tips and demineralization outpaces remineralization, enamel begins to show subtle signs of the ongoing struggle. Recognizing these early indicators can be important, as it signals that the protective mechanisms might be under strain.
One of the earliest visual cues of mineral loss is the appearance of white spot lesions. These are chalky, opaque areas on the enamel surface, often seen along the gumline or around orthodontic brackets. The change in appearance is due to the subsurface enamel becoming more porous as minerals are leached out, altering the way light reflects off the tooth. At this stage, the surface layer may still be largely intact, and the process is often reversible with improved conditions for remineralization.
As enamel thins or demineralizes further, tooth sensitivity can develop. The enamel normally insulates the underlying dentin, which contains microscopic tubules leading to the tooth’s nerve. When enamel is compromised, stimuli like hot, cold, sweet, or acidic foods and drinks can more easily reach the dentin, triggering a short, sharp sensation.
In more advanced stages of enamel wear, particularly from erosion, the teeth might appear more translucent, especially at the incisal edges of the front teeth. This occurs because the enamel layer thins, allowing the yellowish color of the underlying dentin to show through more prominently, or the edges themselves become almost see-through. The overall shape of the teeth can also change, with surfaces appearing smoother, more rounded, or even cupped out in areas of significant erosion.
Supporting Enamel’s Natural Resilience
While dental enamel is inherently strong and has natural repair mechanisms, there are several general practices and awareness points that can help support its ongoing battle against acid attacks. The focus is on fostering an oral environment that favors remineralization over demineralization.
Dietary Awareness: Being mindful of the frequency and type of acidic foods and sugary snacks consumed is a fundamental aspect. This does not necessarily mean eliminating all such items, but rather considering how often the teeth are exposed. For instance, consuming a sugary drink quickly rather than sipping it over a long period limits the duration of the acid challenge. Rinsing the mouth with plain water after consuming acidic or sugary items can also help to neutralize and clear residual acids and sugars.
The Importance of Hydration: Staying well-hydrated is crucial for maintaining healthy salivary flow. Since saliva is a primary defender against acid, ensuring its ample production supports all its protective functions, from buffering acids to supplying minerals for repair.
Gentle but Thorough Cleaning: Regular and effective removal of dental plaque is vital to reduce the concentration of acid-producing bacteria on tooth surfaces. Using a soft-bristled toothbrush and a gentle technique helps prevent abrasive wear on the enamel while still effectively cleaning the teeth. Cleaning between teeth, where plaque can easily accumulate, is also an important part of this routine.
Understanding Fluoride’s Role: Fluoride is widely recognized for its benefits in strengthening enamel and promoting remineralization. It is a common ingredient in many commercially available toothpastes and mouth rinses. Its presence in the oral cavity helps to create a more acid-resistant enamel surface (fluorapatite) and aids in the repair of early demineralized lesions. Consistent, low-level exposure to fluoride is generally considered beneficial for maintaining enamel health.
It is important to remember that while these practices support enamel health, significant concerns about tooth wear or sensitivity should always be discussed with a dental professional. They can provide personalized advice and identify any underlying issues that may require specific attention. This information is for general understanding and not a substitute for professional guidance. Always consult a qualified expert for dental health matters.
The Enduring Strength of Enamel
Dental enamel, the body’s hardest tissue, is a marvel of biological engineering, designed to withstand significant daily challenges. Its crystalline structure provides immense strength, yet it remains in a constant, dynamic interplay with its environment, particularly susceptible to the subtle but persistent threat of acid attack. The balance between demineralization and remineralization dictates its fate.
Through the remarkable actions of saliva and the beneficial incorporation of minerals like fluoride, enamel has powerful allies in its defense. Understanding the nature of acid challenges, the mechanisms of enamel protection, and the factors that can tip the scales allows for a greater appreciation of this resilient material. Supporting its natural defenses through mindful habits contributes to the long-term integrity of this vital protective layer, allowing our teeth to continue their essential work for years to come.