Ever crunched down on something unexpectedly hard, like an unpopped popcorn kernel, and marveled that your teeth didn’t shatter? That remarkable resilience comes courtesy of human enamel, the hardest substance in your body. It’s the gleaming, protective outer layer of our teeth, and its strength is a fascinating subject when you start comparing it to other materials, both natural and man-made. While it might not win every strongman competition against industrial materials, its specific blend of properties makes it perfectly suited for its lifelong job of biting, tearing, and grinding food.
Understanding Material Strength
Before we dive into comparisons, it’s helpful to understand that “strength” isn’t a single, simple measure. Materials scientists look at several characteristics. Hardness is one, often measured on the Mohs scale, which ranks materials based on their ability to scratch one another. Then there’s compressive strength, how much force a material can withstand being squeezed before it deforms or breaks. Tensile strength is its ability to resist being pulled apart. And crucially, especially for something like teeth, there’s fracture toughness – a material’s resistance to crack propagation. A very hard material can sometimes be quite brittle, meaning it shatters easily once a crack starts.
The Profile of Human Enamel
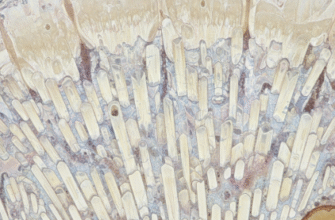
Human enamel is a true marvel of biological engineering. Its primary component, making up about 96% of its mass, is a mineral called hydroxyapatite, a crystalline calcium phosphate. These crystals are organized into incredibly fine, long, densely packed structures called enamel rods or prisms. Imagine millions of tiny, tightly bundled crystalline straws running roughly perpendicular from the underlying dentin to the tooth surface. This highly organized, hierarchical structure is key to its properties.
On the Mohs scale of mineral hardness, enamel typically scores around a 5. To put that into perspective, talc is a 1 (very soft, easily scratched by a fingernail), a fingernail itself is about 2.5, and diamond is a 10 (the hardest known natural material). So, enamel is quite hard, harder than common metals like iron or copper in their pure forms. However, it’s not just raw hardness. The small amount of organic material (proteins like amelogenins) and water within the enamel structure, particularly between the rods, is thought to play a role in preventing cracks from spreading too easily, adding a degree of toughness that pure, brittle hydroxyapatite wouldn’t have on its own. This interplay between hardness and toughness is what makes enamel so effective for its daily grind.
Scientific analysis confirms that human enamel is predominantly composed of hydroxyapatite crystals. These crystals are meticulously arranged into microscopic rod-like structures, often called prisms. This intricate architecture is fundamental to enamel’s remarkable ability to withstand the daily forces of mastication over a lifetime.
Enamel Versus the World: A Strength Showdown
Now for the fun part: how does our dental armor fare against other materials we encounter or use? It’s important to remember that different materials are designed for different purposes, so “stronger” often depends on the specific application.
Common Objects and Softer Materials
Let’s start with some everyday benchmarks. As mentioned, your fingernail (Mohs 2.5) is significantly softer than enamel. This is why you can’t scratch your teeth with your nails (though you can certainly dislodge plaque!). Common copper coins or wires sit around Mohs 3 to 3.5, also softer. Even pure iron is typically around Mohs 4 to 4.5, meaning enamel can scratch it. These comparisons highlight that enamel is indeed a robust biological material, outperforming many common substances in terms of surface hardness.
Harder, Tougher, or Just Different?
Things get more interesting when we look at materials known for their resilience.
Glass: Common window or bottle glass typically has a Mohs hardness of around 5.5 to 7, meaning most glass is harder than enamel and can scratch it. However, glass is famously brittle. While it resists surface scratching well, a sharp impact can cause it to shatter catastrophically. Enamel, while also somewhat brittle compared to metals, has a more complex microstructure that offers some resistance to crack propagation, especially with the softer dentin layer beneath it acting as a shock absorber.
Steel: This is a broad category. A simple piece of structural steel might have a Mohs hardness around 4-4.5, but many hardened steel alloys, like those used in tools or knife blades, can range from Mohs 5.5 up to 8.5. These steels are significantly harder than enamel and also possess much greater fracture toughness. You can hit a steel hammer with great force, and it won’t chip like enamel might. However, steel rusts; enamel doesn’t (though it can demineralize due to acid). The design goals are different: steel is for tools and structures, enamel for biological functions in a wet environment.
Granite: A common countertop material, granite is an igneous rock composed mainly of quartz and feldspar. Its Mohs hardness is typically in the 6-7 range. It’s very hard and resistant to scratching, which is why it’s popular in kitchens. It also has good compressive strength. While harder than enamel, granite is also brittle. You can chip a granite countertop with a hard impact, much like you can chip a tooth.
Quartz: One of the main components of granite, pure quartz has a Mohs hardness of 7. This makes it definitively harder than enamel. If you were to rub a quartz crystal against a tooth, the tooth would be scratched. This is why very abrasive toothpastes (which historically sometimes contained silica, a form of quartz) could wear down enamel over time.
Diamond: At Mohs 10, diamond is the undisputed champion of mineral hardness. It can scratch any other material on the scale, including enamel, with ease. Dental tools tipped with diamond are used to cut and shape teeth precisely because of this extreme hardness difference.
Specialized Strength: Toughness and Resilience
Hardness isn’t everything. Some materials are famed for other types of strength.
Spider Silk: On a weight-for-weight basis, some types of spider silk have a tensile strength comparable to, or even exceeding, high-grade steel. It’s incredibly tough, meaning it can absorb a lot of energy before breaking. Enamel, by contrast, has relatively low tensile strength and is not “stretchy” or energy-absorbent in the same way. They are designed for entirely different purposes.
Wood: Wood is an anisotropic material, meaning its strength properties vary depending on the direction of the grain. It has good tensile strength along the grain and decent compressive strength. Its main advantage is its toughness and ability to flex before breaking. While much softer than enamel (you can easily dent wood with your teeth), its structural resilience is notable for a natural material. Enamel needs to be rigid; wood benefits from some flexibility.
Man-Made Dental Materials
Dentists use various materials to repair or replace tooth structure, and their properties are carefully chosen to mimic or complement enamel.
Porcelain/Ceramics: Dental porcelains are designed to be aesthetically pleasing and wear-resistant. Their Mohs hardness can be similar to or even slightly higher than enamel, often in the 5-7 range depending on the specific formulation. Like enamel, they can be quite hard but also brittle if not properly supported or if subjected to excessive force. Modern dental ceramics are constantly being improved for better fracture toughness.
Composite Resins: These tooth-colored filling materials are a mixture of a resin matrix and inorganic filler particles (like silica or glass). They are generally softer than enamel, with Mohs hardness typically ranging from 3 to 5. This means they can wear down more quickly than enamel or porcelain, but they bond well to tooth structure and are more easily repaired. Their lower stiffness can also be an advantage in some situations, as they can flex slightly with the tooth.
Amalgam: Traditional silver fillings are made from a metal alloy. Amalgam is very strong in compression and quite durable, often lasting for many years. Its hardness is comparable to enamel, but its wear characteristics and appearance are different. It doesn’t bond to the tooth; it’s packed in.
What Makes Enamel Uniquely Suited for Its Job?
While enamel might be outclassed in pure hardness by quartz or diamond, or in toughness by steel or spider silk, it possesses a unique combination of properties ideal for its biological role. Its hardness provides excellent wear resistance against the wide variety of foods we consume. Its highly mineralized nature makes it resistant to the mechanical stresses of chewing.
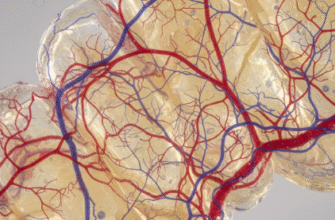
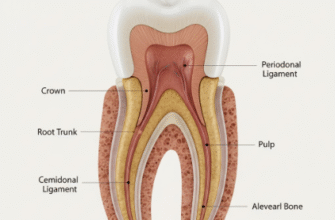
Crucially, enamel doesn’t exist in isolation. It’s bonded to the underlying dentin, which is a softer, more elastic, and more fracture-resistant material (Mohs hardness around 3-4). This dentin layer acts like a shock absorber, cushioning the more brittle enamel from impact forces and helping to prevent cracks from propagating through the entire tooth. This composite structure (hard enamel over tougher dentin) is a brilliant piece of natural design, optimizing for both wear resistance and fracture prevention.
Furthermore, enamel has a limited capacity for remineralization. Saliva contains calcium and phosphate ions that can help repair microscopic lesions caused by early acid attack, essentially a form of self-healing, though this process cannot replace lost enamel from significant decay or trauma.
The Achilles’ Heel: Vulnerabilities of Enamel
Despite its impressive strength, enamel is not invincible. Its primary weakness is its susceptibility to acid demineralization. Acids produced by bacteria metabolizing sugars, or from acidic foods and drinks, can dissolve the hydroxyapatite crystals. This is the process that leads to dental caries (cavities). Once enamel is lost through decay, wear, or trauma, the body cannot regenerate it. This is why preserving the enamel we have is so critical through good oral hygiene and a sensible diet.
While hard, enamel is still brittle enough that it can chip or crack under sudden, sharp impacts or excessive force, such as biting down on an olive pit or from habits like ice chewing or teeth grinding (bruxism).
Conclusion: A Biological Masterpiece
Human enamel stands as a testament to nature’s ingenuity. While it might not top the charts in every single strength category when pitted against specialized industrial materials, its specific combination of high hardness for wear resistance, coupled with the supportive underlying dentin that enhances its functional toughness, makes it exceptionally well-suited for the demanding, lifelong task of processing food. It’s a durable, resilient, and biologically compatible material that performs admirably under a wide range of conditions. Understanding its strengths and vulnerabilities helps us appreciate just how remarkable this natural coating truly is, reminding us to take good care of it.